miR-122-5p modulates the radiosensitivity of cervical cancer cells by regulating cell division cycle 25A (CDC25A)
- PMID: 31505105
- PMCID: PMC6823283
- DOI: 10.1002/2211-5463.12730
miR-122-5p modulates the radiosensitivity of cervical cancer cells by regulating cell division cycle 25A (CDC25A)
Abstract
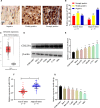
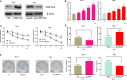
Cervical cancer is one of the most common gynecological malignancies globally, Unfortunately, radiotherapy and chemotherapy are not effective at treating some cases of this disease, and the 5-year survival rate is only 40-50%. Cell division cycle 25A (CDC25A) has been shown to induce radioresistance in a variety of tumor cells, but the role of CDC25A in the radioresistance of cervical cancer has not been fully elucidated. Here, we report that CDC25A is highly expressed and miR-122-5p lowly expressed in cervical cancer tissues and cells. The TargetScan database was used to predict CDC25A as a target of miR-122-5p, and the interactions between miR-122-5p and CDC25A were further confirmed by western blot, real-time PCR and dual-luciferase reporter assay. Under X-ray irradiation, up-regulation of CDC25A can promote the radiation resistance of cervical cancer cells, whereas overexpression of miR-122-5p or knockdown of CDC25A inhibits the survival and induces apoptosis of cervical cancer colonies. In conclusion, our data suggest that miR-122-5p enhances the radiosensitivity of cervical cancer cells by targeting CDC25A.
Keywords: CDC25A; cervical cancer; miR-122-5p; radiosensitivity.
© 2019 The Authors. Published by FEBS Press and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
LINC00662 contributes to the progression and the radioresistance of cervical cancer by regulating miR-497-5p and CDC25A.Cell Biochem Funct. 2020 Dec;38(8):1139-1151. doi: 10.1002/cbf.3580. Epub 2020 Sep 1. Cell Biochem Funct. 2020. PMID: 32869878
-
MicroRNA-99a-5p suppresses breast cancer progression and cell-cycle pathway through downregulating CDC25A.J Cell Physiol. 2019 Apr;234(4):3526-3537. doi: 10.1002/jcp.26906. Epub 2018 Nov 15. J Cell Physiol. 2019. PMID: 30443946
-
MiR-99a-5p Constrains Epithelial-Mesenchymal Transition of Cervical Squamous Cell Carcinoma Via Targeting CDC25A/IL6.Mol Biotechnol. 2022 Nov;64(11):1234-1243. doi: 10.1007/s12033-022-00496-y. Epub 2022 May 9. Mol Biotechnol. 2022. PMID: 35532870
-
MiR-365 enhances the radiosensitivity of non-small cell lung cancer cells through targeting CDC25A.Biochem Biophys Res Commun. 2019 Apr 30;512(2):392-398. doi: 10.1016/j.bbrc.2019.03.082. Epub 2019 Mar 20. Biochem Biophys Res Commun. 2019. PMID: 30902389
-
miR-34a-5p blocks cervical cancer growth and migration by downregulating CDC25A.J BUON. 2021 Sep-Oct;26(5):1768-1774. J BUON. 2021. PMID: 34761581
Cited by
-
The circCDK17/miR-122-5p/ASF1B axis regulates the progression of cervical cancer.Histol Histopathol. 2023 Mar;38(3):359-371. doi: 10.14670/HH-18-527. Epub 2022 Sep 30. Histol Histopathol. 2023. PMID: 36178207
-
Role of miR-100-5p and CDC25A in breast carcinoma cells.PeerJ. 2022 Jan 3;9:e12263. doi: 10.7717/peerj.12263. eCollection 2022. PeerJ. 2022. PMID: 35036112 Free PMC article.
-
Circadian Rhythm Dysregulation and Leukemia Development: The Role of Clock Genes as Promising Biomarkers.Int J Mol Sci. 2022 Jul 26;23(15):8212. doi: 10.3390/ijms23158212. Int J Mol Sci. 2022. PMID: 35897788 Free PMC article. Review.
-
Oncogenic role of ALX3 in cervical cancer cells through KDM2B-mediated histone demethylation of CDC25A.BMC Cancer. 2021 Jul 16;21(1):819. doi: 10.1186/s12885-021-08552-7. BMC Cancer. 2021. PMID: 34266408 Free PMC article.
-
CDC25A inhibition suppresses cell proliferation and induces G1/S‑phase cell cycle arrest in nasopharyngeal carcinoma.Mol Med Rep. 2023 May;27(5):109. doi: 10.3892/mmr.2023.12996. Epub 2023 Apr 13. Mol Med Rep. 2023. PMID: 37052240 Free PMC article.

