Cross-Talk between Transforming Growth Factor-β and Periostin Can Be Targeted for Pulmonary Fibrosis
- PMID: 31505128
- PMCID: PMC6993541
- DOI: 10.1165/rcmb.2019-0245OC
Cross-Talk between Transforming Growth Factor-β and Periostin Can Be Targeted for Pulmonary Fibrosis
Abstract
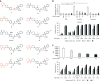
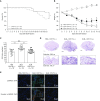
Idiopathic pulmonary fibrosis (IPF) is a devastating disease characterized as progressive and irreversible fibrosis in the interstitium of lung tissues. There is still an unmet need to develop a novel therapeutic drug for IPF. We have previously demonstrated that periostin, a matricellular protein, plays an important role in the pathogenesis of pulmonary fibrosis. However, the underlying mechanism of how periostin causes pulmonary fibrosis remains unclear. In this study, we sought to learn whether the cross-talk between TGF-β (transforming growth factor-β), a central mediator in pulmonary fibrosis, and periostin in lung fibroblasts leads to generation of pulmonary fibrosis and whether inhibitors for integrin αVβ3, a periostin receptor, can block pulmonary fibrosis in model mice and the TGF-β signals in fibroblasts from patients with IPF. We found that cross-talk exists between TGF-β and periostin signals via αVβ3/β5 converging into Smad3. This cross-talk is necessary for the expression of TGF-β downstream effector molecules important for pulmonary fibrosis. Moreover, we identified several potent integrin low-molecular-weight inhibitors capable of blocking cross-talk with TGF-β signaling. One of the compounds, CP4715, attenuated bleomycin-induced pulmonary fibrosis in vivo in mice and the TGF-β signals in vitro in fibroblasts from patients with IPF. These results suggest that the cross-talk between TGF-β and periostin can be targeted for pulmonary fibrosis and that CP4715 can be a potential therapeutic agent to block this cross-talk.
Keywords: idiopathic pulmonary fibrosis; inhibitor; integrin; periostin; transforming growth factor-β.
Figures






Similar articles
-
Periostin plays a critical role in the cell cycle in lung fibroblasts.Respir Res. 2020 Jan 30;21(1):38. doi: 10.1186/s12931-020-1299-0. Respir Res. 2020. PMID: 32000779 Free PMC article.
-
Protein tyrosine phosphatase-α amplifies transforming growth factor-β-dependent profibrotic signaling in lung fibroblasts.Am J Physiol Lung Cell Mol Physiol. 2020 Aug 1;319(2):L294-L311. doi: 10.1152/ajplung.00235.2019. Epub 2020 Jun 3. Am J Physiol Lung Cell Mol Physiol. 2020. PMID: 32491951 Free PMC article.
-
Periostin promotes fibrosis and predicts progression in patients with idiopathic pulmonary fibrosis.Am J Physiol Lung Cell Mol Physiol. 2012 Dec 15;303(12):L1046-56. doi: 10.1152/ajplung.00139.2012. Epub 2012 Oct 5. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 23043074 Free PMC article.
-
Ability of Periostin as a New Biomarker of Idiopathic Pulmonary Fibrosis.Adv Exp Med Biol. 2019;1132:79-87. doi: 10.1007/978-981-13-6657-4_9. Adv Exp Med Biol. 2019. PMID: 31037627 Review.
-
Sphingolipids in pulmonary fibrosis.Adv Biol Regul. 2015 Jan;57:55-63. doi: 10.1016/j.jbior.2014.09.008. Epub 2014 Oct 13. Adv Biol Regul. 2015. PMID: 25446881 Free PMC article. Review.
Cited by
-
Periostin secreted by activated fibroblasts in idiopathic pulmonary fibrosis promotes tumorigenesis of non-small cell lung cancer.Sci Rep. 2021 Oct 26;11(1):21114. doi: 10.1038/s41598-021-00717-5. Sci Rep. 2021. PMID: 34702952 Free PMC article.
-
Expression of the Pro-Fibrotic Marker Periostin in a Mouse Model of Duchenne Muscular Dystrophy.Biomedicines. 2024 Jan 18;12(1):216. doi: 10.3390/biomedicines12010216. Biomedicines. 2024. PMID: 38255321 Free PMC article.
-
Possible Roles of Periostin in the Formation of Hemodialysis Vascular Access Stenosis after Polytetrafluoroethylene Graft Implantation in Dogs.Int J Mol Sci. 2020 May 4;21(9):3251. doi: 10.3390/ijms21093251. Int J Mol Sci. 2020. PMID: 32375347 Free PMC article.
-
Overlapping Systemic Proteins in COVID-19 and Lung Fibrosis Associated with Tissue Remodeling and Inflammation.Biomedicines. 2024 Dec 19;12(12):2893. doi: 10.3390/biomedicines12122893. Biomedicines. 2024. PMID: 39767799 Free PMC article.
-
Periostin and rheumatic diseases: early insights from a systematic review and meta-analysis.Clin Exp Med. 2025 Mar 7;25(1):75. doi: 10.1007/s10238-025-01615-0. Clin Exp Med. 2025. PMID: 40053143 Free PMC article.
References
-
- Martinez FJ, Collard HR, Pardo A, Raghu G, Richeldi L, Selman M, et al. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers. 2017;3:17074. - PubMed
-
- Ask K, Martin GE, Kolb M, Gauldie J. Targeting genes for treatment in idiopathic pulmonary fibrosis: challenges and opportunities, promises and pitfalls. Proc Am Thorac Soc. 2006;3:389–393. - PubMed
-
- Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12:325–338. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

