Updated mechanisms underlying sickle cell disease-associated pain
- PMID: 31505241
- PMCID: PMC6815235
- DOI: 10.1016/j.neulet.2019.134471
Updated mechanisms underlying sickle cell disease-associated pain
Abstract
Sickle cell disease (SCD) is one of the most common severe genetic diseases around the world. A majority of SCD patients experience intense pain, leading to hospitalization, and poor quality of life. Opioids form the bedrock of pain management, but their long-term use is associated with severe side effects including hyperalgesia, tolerance and addiction. Recently, excellent research has shown some new potential mechanisms that underlie SCD-associated pain. This review focused on how transient receptor potential vanilloid 1, endothelin-1/endothelin type A receptor, and cannabinoid receptors contributed to the pathophysiology of SCD-associated pain. Understanding these mechanisms may open a new avenue in managing SCD-associated pain and improving quality of life for SCD patients.
Keywords: Cannabinoid receptors; Endothelin type A receptor; Endothelin-1; Pain; Sickle cell disease; TRPV1.
Copyright © 2019 Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interests
The authors declare no competing interests.
Figures
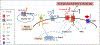
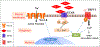
References
-
- Kauf TL, Coates TD, Huazhi L, Mody-Patel N, Hartzema AG, The cost of health care for children and adults with sickle cell disease, Am. J. Hematol 84 (2009) 323–327. - PubMed
-
- Hassell KL, Population estimates of sickle cell disease in the U.S, Am. J. Prev. Med 38 (2010) S512–S521. - PubMed
-
- PAULING L, ITANO HA, Sickle cell anemia, a molecular disease, Science 109 (1949) 443. - PubMed
-
- INGRAM VM, A specific chemical difference between the globins of normal human and sickle-cell anaemia haemoglobin, Nature 178 (1956) 792–794. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

