Structural characterization of the PCV2d virus-like particle at 3.3 Å resolution reveals differences to PCV2a and PCV2b capsids, a tetranucleotide, and an N-terminus near the icosahedral 3-fold axes
- PMID: 31505320
- PMCID: PMC6958667
- DOI: 10.1016/j.virol.2019.09.001
Structural characterization of the PCV2d virus-like particle at 3.3 Å resolution reveals differences to PCV2a and PCV2b capsids, a tetranucleotide, and an N-terminus near the icosahedral 3-fold axes
Abstract
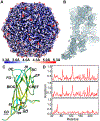
Porcine circovirus 2 (PCV2) has a major impact on the swine industry. Eight PCV2 genotypes (a-h) have been identified using capsid sequence analysis. PCV2d has been designated as the emerging genotype. The cryo-electron microscopy molecular envelope of PCV2d virus-like particles identifies differences between PCV2a, b and d genotypes that accompany the emergence of PCV2b from PCV2a, and PCV2d from PCV2b. These differences indicate that sequence analysis of genotypes is insufficient, and that it is important to determine the PCV2 capsid structure as the virus evolves. Structure-based sequence comparison demonstrate that each genotype possesses a unique combination of amino acids located on the surface of the capsid that undergo substitution. We also demonstrate that the capsid N-terminus moves in response to increasing amount of nucleic acid packaged into the capsid. Furthermore, we model a tetranucleotide between the 5- and 2-fold axes of symmetry that appears to be responsible for capsid stability.
Keywords: Capsid structure; Circovirus; Cryo-electron microscopy; Virus evolution; Virus-like particle (VLP).
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures


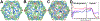


Similar articles
-
Neutralization Mechanism of a Monoclonal Antibody Targeting a Porcine Circovirus Type 2 Cap Protein Conformational Epitope.J Virol. 2020 Apr 16;94(9):e01836-19. doi: 10.1128/JVI.01836-19. Print 2020 Apr 16. J Virol. 2020. PMID: 32075932 Free PMC article.
-
First construction of infectious clone for newly emerging mutation porcine circovirus type 2 (PCV2) followed by comparison with PCV2a and PCV2b genotypes in biological characteristics in vitro.Virol J. 2011 Jun 10;8:291. doi: 10.1186/1743-422X-8-291. Virol J. 2011. PMID: 21658280 Free PMC article.
-
A chimeric virus created by DNA shuffling of the capsid genes of different subtypes of porcine circovirus type 2 (PCV2) in the backbone of the non-pathogenic PCV1 induces protective immunity against the predominant PCV2b and the emerging PCV2d in pigs.Virology. 2016 Nov;498:82-93. doi: 10.1016/j.virol.2016.08.011. Epub 2016 Aug 24. Virology. 2016. PMID: 27564544
-
Genotypic diversity of porcine circovirus type 2 (PCV2) and genotype shift to PCV2d in Korean pig population.Virus Res. 2017 Jan 15;228:24-29. doi: 10.1016/j.virusres.2016.11.015. Epub 2016 Nov 17. Virus Res. 2017. PMID: 27867029
-
Porcine Circovirus Type 2 (PCV2) Vaccines in the Context of Current Molecular Epidemiology.Viruses. 2017 May 6;9(5):99. doi: 10.3390/v9050099. Viruses. 2017. PMID: 28481275 Free PMC article. Review.
Cited by
-
Synthesis and characterization of molecularly imprinted polymer nanoparticles against porcine circovirus type 2 viral-like particles.Anal Bioanal Chem. 2024 Dec;416(30):7357-7368. doi: 10.1007/s00216-024-05576-3. Epub 2024 Oct 11. Anal Bioanal Chem. 2024. PMID: 39392505 Free PMC article.
-
Evidence of Porcine Circovirus Type 2 (PCV2) Genetic Shift from PCV2b to PCV2d Genotype in Sardinia, Italy.Viruses. 2023 Oct 26;15(11):2157. doi: 10.3390/v15112157. Viruses. 2023. PMID: 38005836 Free PMC article.
-
Structural insight into the type-specific epitope of porcine circovirus type 3.Biosci Rep. 2020 Jun 26;40(6):BSR20201109. doi: 10.1042/BSR20201109. Biosci Rep. 2020. PMID: 32458997 Free PMC article.
-
Amino acid mutations of porcine circovirus type 2 (PCV2) capsid protein increase virus binding to host and evade immune responses: An evaluation of viral evolution.Virulence. 2025 Dec;16(1):2535472. doi: 10.1080/21505594.2025.2535472. Epub 2025 Jul 23. Virulence. 2025. PMID: 40699883 Free PMC article.
-
Constant pH molecular dynamics of porcine circovirus 2 capsid protein reveals a mechanism for capsid assembly.Phys Chem Chem Phys. 2021 Nov 10;23(43):24617-24626. doi: 10.1039/d1cp02874j. Phys Chem Chem Phys. 2021. PMID: 34726674 Free PMC article.
References
-
- Afonine PA, Headd JJ, Terwilliger TC, Adams PC, 2013. Computational Crystallography Newsletter 4, 43–44.
-
- Beach NM, Meng XJ, 2012. Efficacy and future prospects of commercially available and experimental vaccines against porcine circovirus type 2 (PCV2). Virus Res 164, 33–42. - PubMed
-
- Cai L, Ni J, Xia Y, Zi Z, Ning K, Qiu P, Li X, Wang B, Liu Q, Hu D, Yu X, Zhou Z, Zhai X, Han X, Tian K, 2012. Identification of an emerging recombinant cluster in porcine circovirus type 2. Virus Res 165, 95–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

