Glucocorticoid regulation of amino acid transport in primary human trophoblast cells
- PMID: 31505460
- PMCID: PMC6872941
- DOI: 10.1530/JME-19-0183
Glucocorticoid regulation of amino acid transport in primary human trophoblast cells
Abstract
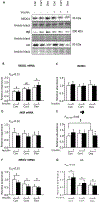
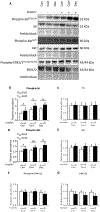
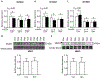
Excess maternal glucocorticoids reduce placental amino acid transport and fetal growth, but whether these effects are mediated directly on the syncytiotrophoblast remains unknown. We hypothesised that glucocorticoids inhibit mechanistic target of rapamycin (mTOR) signaling and insulin-stimulated System A amino acid transport activity in primary human trophoblast (PHT) cells. Syncytialised PHTs, isolated from term placentas (n = 15), were treated with either cortisol (1 μM) or dexamethasone (1 μM), ± insulin (1 nM) for 24 h. Compared to vehicle, dexamethasone increased mRNA expression, but not protein abundance of the mTOR suppressor, regulated in development and DNA damage response 1 (REDD1). Dexamethasone enhanced insulin receptor abundance, activated mTOR complex 1 and 2 signaling and stimulated System A activity, measured by Na+-dependent 14C-methylaminoisobutyric acid uptake. Cortisol also activated mTORC1 without significantly altering insulin receptor or mTORC2 read-outs or System A activity. Both glucocorticoids downregulated expression of the glucocorticoid receptor and the System A transporter genes SLC38A1, SLC38A2 and SLC38A4, without altering SNAT1 or SNAT4 protein abundance. Neither cortisol nor dexamethasone affected System L amino acid transport. Insulin further enhanced mTOR and System A activity, irrespective of glucocorticoid treatment and despite downregulating its own receptor. Contrary to our hypothesis, glucocorticoids do not inhibit mTOR signaling or cause insulin resistance in cultured PHT cells. We speculate that glucocorticoids stimulate System A activity in PHT cells by activating mTOR signaling, which regulates amino acid transporters post-translationally. We conclude that downregulation of placental nutrient transport in vivo following excess maternal glucocorticoids is not mediated by a direct effect on the placenta.
Keywords: fetal development; maternal–fetal exchange; mechanistic target of rapamycin; placenta; solute carrier family 38A members 1, 2 and 4.
Conflict of interest statement
DECLARATION OF INTEREST
The authors declare no conflict of interest
Figures
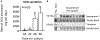

Similar articles
-
Apelin is a novel regulator of human trophoblast amino acid transport.Am J Physiol Endocrinol Metab. 2019 May 1;316(5):E810-E816. doi: 10.1152/ajpendo.00012.2019. Epub 2019 Mar 5. Am J Physiol Endocrinol Metab. 2019. PMID: 30835509 Free PMC article.
-
Regulation of amino acid transporters by glucose and growth factors in cultured primary human trophoblast cells is mediated by mTOR signaling.Am J Physiol Cell Physiol. 2009 Sep;297(3):C723-31. doi: 10.1152/ajpcell.00191.2009. Epub 2009 Jul 8. Am J Physiol Cell Physiol. 2009. PMID: 19587219
-
Mammalian target of rapamycin in the human placenta regulates leucine transport and is down-regulated in restricted fetal growth.J Physiol. 2007 Jul 1;582(Pt 1):449-59. doi: 10.1113/jphysiol.2007.129676. Epub 2007 Apr 26. J Physiol. 2007. PMID: 17463046 Free PMC article.
-
The emerging role of mTORC1 signaling in placental nutrient-sensing.Placenta. 2012 Nov;33 Suppl 2(Suppl 2):e23-9. doi: 10.1016/j.placenta.2012.05.010. Epub 2012 Jun 10. Placenta. 2012. PMID: 22687819 Free PMC article. Review.
-
Placental regulation of fetal nutrient supply.Curr Opin Clin Nutr Metab Care. 2013 May;16(3):292-7. doi: 10.1097/MCO.0b013e32835e3674. Curr Opin Clin Nutr Metab Care. 2013. PMID: 23416721 Review.
Cited by
-
Dexamethasone acutely suppresses the anabolic SNAT2/SLC38A2 amino acid transporter protein in L6-G8C5 rat skeletal muscle cells.FASEB Bioadv. 2020 Oct 21;3(1):36-48. doi: 10.1096/fba.2020-00076. eCollection 2021 Jan. FASEB Bioadv. 2020. PMID: 33490882 Free PMC article.
-
miR-373-3p Regulates the Proliferative and Migratory Properties of Human HTR8 Cells via SLC38A1 Modulation.Dis Markers. 2022 Jun 28;2022:6582357. doi: 10.1155/2022/6582357. eCollection 2022. Dis Markers. 2022. PMID: 35837487 Free PMC article.
-
Regulation of placental amino acid transport in health and disease.Acta Physiol (Oxf). 2024 Jul;240(7):e14157. doi: 10.1111/apha.14157. Epub 2024 May 6. Acta Physiol (Oxf). 2024. PMID: 38711335 Free PMC article. Review.
-
Metabolic Consequences of Glucocorticoid Exposure before Birth.Nutrients. 2022 May 30;14(11):2304. doi: 10.3390/nu14112304. Nutrients. 2022. PMID: 35684104 Free PMC article. Review.
-
The stress-responsive protein REDD1 and its pathophysiological functions.Exp Mol Med. 2023 Sep;55(9):1933-1944. doi: 10.1038/s12276-023-01056-3. Epub 2023 Sep 1. Exp Mol Med. 2023. PMID: 37653030 Free PMC article. Review.
References
-
- AUDETTE MC, CHALLIS JR, JONES RL, SIBLEY CP & MATTHEWS SG 2011. Antenatal Dexamethasone Treatment in Midgestation Reduces System A-Mediated Transport in the Late-Gestation Murine Placenta. Endocrinology, 152, 3561–70. - PubMed
-
- AUDETTE MC, CHALLIS JRG, JONES RL, SIBLEY CP & MATTHEWS SG 2014. Synthetic Glucocorticoid Reduces Human Placental System A Transport in Women Treated With Antenatal Therapy. The Journal of Clinical Endocrinology & Metabolism, 99, E2226–E2233. - PubMed
-
- AUDETTE MC, GREENWOOD SL, SIBLEY CP, JONES CJP, CHALLIS JRG, MATTHEWS SG & JONES RL 2010. Dexamethasone stimulates placental system A transport and trophoblast differentiation in term villous explants. Placenta, 31, 97–105. - PubMed
-
- BALLARD PL & BALLARD RA 1995. Scientific basis and therapeutic regimens for use of antenatal glucocorticoids. Am J Obstet Gynecol, 173, 254–62. - PubMed
-
- CRAMER S, BEVERIDGE M, KILBERG M & NOVAK D 2002. Physiological importance of system A-mediated amino acid transport to rat fetal development. Am J Physiol Cell Physiol, 282, C153–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

