Could changing the course of Alzheimer's disease pathology with immunotherapy prevent dementia?
- PMID: 31505546
- PMCID: PMC6933504
- DOI: 10.1093/brain/awz165
Could changing the course of Alzheimer's disease pathology with immunotherapy prevent dementia?
Abstract
This scientific commentary refers to ‘Persistent neuropathological effects 14 years following amyloid-β immunization in Alzheimer’s disease’ by Nicoll et al. (doi:
Figures
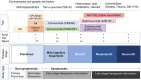
Comment on
-
Persistent neuropathological effects 14 years following amyloid-β immunization in Alzheimer's disease.Brain. 2019 Jul 1;142(7):2113-2126. doi: 10.1093/brain/awz142. Brain. 2019. PMID: 31157360 Free PMC article.
References
-
- Bayer AJ, Bullock R, Jones RW, Wilkinson D, Paterson KR, Jenkins L, et al.Evaluation of the safety and immunogenicity of synthetic Abeta42 (AN1792) in patients with AD. Neurology 2005; 64: 94–101. - PubMed
-
- Boche D, Denham N, Holmes C, Nicoll JA. Neuropathology after active Abeta42 immunotherapy: implications for Alzheimer’s disease pathogenesis. Acta Neuropathol 2010; 120: 369–84. - PubMed
-
- Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, et al.Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology 2005; 64: 1553–62. - PubMed
-
- Masliah E, Hansen L, Adame A, Crews L, Bard F, Lee C, et al.Abeta vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology 2005; 64: 129–31. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

