Freezing of gait in Parkinson's disease reflects a sudden derangement of locomotor network dynamics
- PMID: 31505548
- PMCID: PMC6598629
- DOI: 10.1093/brain/awz141
Freezing of gait in Parkinson's disease reflects a sudden derangement of locomotor network dynamics
Abstract
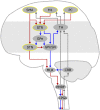
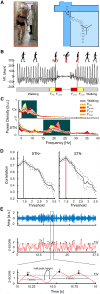
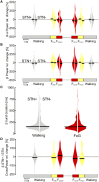
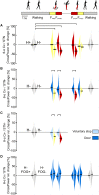
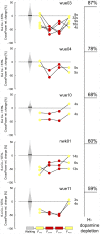
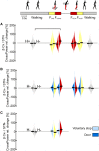
Freezing of gait is a disabling symptom of Parkinson's disease that causes a paroxysmal inability to generate effective stepping. The underlying pathophysiology has recently migrated towards a dysfunctional supraspinal locomotor network, but the actual network derangements during ongoing gait freezing are unknown. We investigated the communication between the cortex and the subthalamic nucleus, two main nodes of the locomotor network, in seven freely-moving subjects with Parkinson's disease with a novel deep brain stimulation device, which allows on-demand recording of subthalamic neural activity from the chronically-implanted electrodes months after the surgical procedure. Multisite neurophysiological recordings during (effective) walking and ongoing gait freezing were combined with kinematic measurements and individual molecular brain imaging studies. Patients walked in a supervised environment closely resembling everyday life challenges. We found that during (effective) walking, the cortex and subthalamic nucleus were synchronized in a low frequency band (4-13 Hz). In contrast, gait freezing was characterized in every patient by low frequency cortical-subthalamic decoupling in the hemisphere with less striatal dopaminergic innervation. Of relevance, this decoupling was already evident at the transition from normal (effective) walking into gait freezing, was maintained during the freezing episode, and resolved with recovery of the effective walking pattern. This is the first evidence for a decoding of the networked processing of locomotion in Parkinson's disease and suggests that freezing of gait is a 'circuitopathy' related to a dysfunctional cortical-subcortical communication. A successful therapeutic approach for gait freezing in Parkinson's disease should aim at directly targeting derangements of neural network dynamics.
Keywords: Parkinson’s disease; basal ganglia; beta oscillations; deep brain stimulation; gait.
© The Author(s) (2019). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures
References
-
- Benda J, Longtin A, Maler L. A synchronization-desynchronization code for natural communication signals. Neuron 2006; 52: 347–58. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

