Impact of 13-Valent Pneumococcal Conjugate Vaccine on Meningitis and Pneumonia Hospitalizations in Children aged <5 Years in Senegal, 2010-2016
- PMID: 31505625
- PMCID: PMC6756160
- DOI: 10.1093/cid/ciz457
Impact of 13-Valent Pneumococcal Conjugate Vaccine on Meningitis and Pneumonia Hospitalizations in Children aged <5 Years in Senegal, 2010-2016
Abstract
Background: Senegal introduced a 13-valent pneumococcal conjugate vaccine (PCV13) in October 2013, given at 6, 10, and 14 weeks of age. We document trends of meningitis and pneumonia after the PCV13 introduction.
Methods: From October 2010-October 2016, hospitalization data for clinical meningitis and pneumonia in children aged <5 years were collected from logbooks at a large, tertiary, pediatric hospital in Dakar. We used a set of predetermined keywords to define hospitalizations for extraction from hospital registers. We conducted a time-series analysis and compared hospitalizations before and after the PCV13 introduction, accounting for seasonality. The initial PCV13 uptake period (October 2013-September 2014) was considered to be transitional and was excluded.
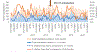
Results: Over the 7-year period, 1836 and 889 hospitalizations with a discharge diagnosis of pneumonia and meningitis, respectively, occurred in children aged <5 years. In children aged <12 months, a small, significant reduction in pneumonia was observed post-PCV13 (-3.8%, 95% confidence interval [CI] -1.5 to -5.9%). No decline was observed among children aged 12-59 months (-0.7%, 95% CI -0.8 to 2.2%). Meningitis hospitalizations remained stable for children aged <12 months (1.8%, 95% CI -0.9 to 4.4%) and 12-59 months (-0.5%, 95% CI -3.6 to 2.6%).
Conclusions: We used data from 1 hospital to detect a small, significant reduction in all-cause pneumonia hospitalizations 2 years post-PCV13 introduction in infants; the same trend was not measurable in children aged 12-59 months or in meningitis cases. There is a need for continued surveillance to assess the long-term impact of sustained PCV13 use and to monitor how pneumococcus is causing disease in the meningitis belt.
Keywords: hospitalization data; meningitis; pneumococcal conjugate vaccine; pneumonia; vaccine impact.
Published by Oxford University Press for the Infectious Diseases Society of America 2019.
Conflict of interest statement
Figures

Similar articles
-
Systematic review on the impact of the pneumococcal conjugate vaccine ten valent (PCV10) or thirteen valent (PCV13) on all-cause, radiologically confirmed and severe pneumonia hospitalisation rates and pneumonia mortality in children 0-9 years old.J Glob Health. 2023 Feb 3;13:05002. doi: 10.7189/jogh.13.05002. J Glob Health. 2023. PMID: 36734192 Free PMC article.
-
Pneumococcal Conjugate Vaccine Impact on Meningitis and Pneumonia Among Children Aged <5 Years-Zimbabwe, 2010-2016.Clin Infect Dis. 2019 Sep 5;69(Suppl 2):S72-S80. doi: 10.1093/cid/ciz462. Clin Infect Dis. 2019. PMID: 31505631 Free PMC article.
-
Declines in Pneumonia and Meningitis Hospitalizations in Children Under 5 Years of Age After Introduction of 10-Valent Pneumococcal Conjugate Vaccine in Zambia, 2010-2016.Clin Infect Dis. 2019 Sep 5;69(Suppl 2):S58-S65. doi: 10.1093/cid/ciz456. Clin Infect Dis. 2019. PMID: 31505628 Free PMC article.
-
Use of administrative records to assess pneumococcal conjugate vaccine impact on pediatric meningitis and pneumonia hospitalizations in Rwanda.Vaccine. 2016 Oct 17;34(44):5321-5328. doi: 10.1016/j.vaccine.2016.08.084. Epub 2016 Sep 14. Vaccine. 2016. PMID: 27639280
-
Effect of pneumococcal conjugate vaccination on pneumococcal carriage in hospitalised children aged 2-59 months in Mongolia: an active pneumonia surveillance programme.Lancet Microbe. 2024 Dec;5(12):100929. doi: 10.1016/S2666-5247(24)00171-X. Epub 2024 Oct 30. Lancet Microbe. 2024. PMID: 39486429
Cited by
-
Impact of 13-valent pneumococcal conjugate vaccine on laboratory-confirmed pneumococcal meningitis and purulent meningitis among children ˂5 years in Cameroon, 2011-2018.PLoS One. 2021 Apr 15;16(4):e0250010. doi: 10.1371/journal.pone.0250010. eCollection 2021. PLoS One. 2021. PMID: 33857235 Free PMC article.
-
Effect of Haemophilus influenzae Type b and 13-Valent Pneumococcal Conjugate Vaccines on Childhood Pneumonia Hospitalizations and Deaths in Botswana.Clin Infect Dis. 2021 Jul 15;73(2):e410-e416. doi: 10.1093/cid/ciaa919. Clin Infect Dis. 2021. PMID: 32634831 Free PMC article.
-
Inequitable Distribution of Global Economic Benefits from Pneumococcal Conjugate Vaccination.Vaccines (Basel). 2024 Jul 12;12(7):767. doi: 10.3390/vaccines12070767. Vaccines (Basel). 2024. PMID: 39066405 Free PMC article.
-
Etiologic Profile of the Pneumococcus in Ghana: A Systematic Review.Biomed Res Int. 2024 Aug 27;2024:8368996. doi: 10.1155/2024/8368996. eCollection 2024. Biomed Res Int. 2024. PMID: 39229361 Free PMC article.
-
Systematic review on the impact of the pneumococcal conjugate vaccine ten valent (PCV10) or thirteen valent (PCV13) on all-cause, radiologically confirmed and severe pneumonia hospitalisation rates and pneumonia mortality in children 0-9 years old.J Glob Health. 2023 Feb 3;13:05002. doi: 10.7189/jogh.13.05002. J Glob Health. 2023. PMID: 36734192 Free PMC article.
References
-
- World Health Organization. Estimated Hib and pneumococcal deaths for children under 5 years of age. Available at: http://www.who.int/immunization/monitoring_surveillance/burden/estimates... Accessed 31 August 2018.
-
- Ba I, Ba A, Faye P, et al. Pediatric invasive pneumococcal disease in Senegal. Med Mal Infect 2015; 45:464–469. - PubMed
-
- World Health Organization. Pneumococcal conjugate vaccine for pneumococcal conjugate vaccines in infants and children under 5 years of age: WHO position paper – February 2019. Wkly Epidemiol Rec 2019; 94(8):85–104.

