A DAO1-Mediated Circuit Controls Auxin and Jasmonate Crosstalk Robustness during Adventitious Root Initiation in Arabidopsis
- PMID: 31505771
- PMCID: PMC6769753
- DOI: 10.3390/ijms20184428
A DAO1-Mediated Circuit Controls Auxin and Jasmonate Crosstalk Robustness during Adventitious Root Initiation in Arabidopsis
Abstract
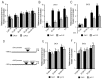
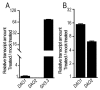
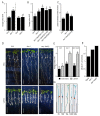
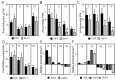
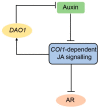
Adventitious rooting is a post-embryonic developmental program governed by a multitude of endogenous and environmental cues. Auxin, along with other phytohormones, integrates and translates these cues into precise molecular signatures to provide a coherent developmental output. Auxin signaling guides every step of adventitious root (AR) development from the early event of cell reprogramming and identity transitions until emergence. We have previously shown that auxin signaling controls the early events of AR initiation (ARI) by modulating the homeostasis of the negative regulator jasmonate (JA). Although considerable knowledge has been acquired about the role of auxin and JA in ARI, the genetic components acting downstream of JA signaling and the mechanistic basis controlling the interaction between these two hormones are not well understood. Here we provide evidence that COI1-dependent JA signaling controls the expression of DAO1 and its closely related paralog DAO2. In addition, we show that the dao1-1 loss of function mutant produces more ARs than the wild type, probably due to its deficiency in accumulating JA and its bioactive metabolite JA-Ile. Together, our data indicate that DAO1 controls a sensitive feedback circuit that stabilizes the auxin and JA crosstalk during ARI.
Keywords: adventitious roots; auxin; auxin oxidation; jasmonates; organogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Jasmonate promotes auxin-induced adventitious rooting in dark-grown Arabidopsis thaliana seedlings and stem thin cell layers by a cross-talk with ethylene signalling and a modulation of xylogenesis.BMC Plant Biol. 2018 Sep 6;18(1):182. doi: 10.1186/s12870-018-1392-4. BMC Plant Biol. 2018. PMID: 30189848 Free PMC article.
-
Auxin controls Arabidopsis adventitious root initiation by regulating jasmonic acid homeostasis.Plant Cell. 2012 Jun;24(6):2515-27. doi: 10.1105/tpc.112.099119. Epub 2012 Jun 22. Plant Cell. 2012. PMID: 22730403 Free PMC article.
-
Jasmonic Acid Inhibits Auxin-Induced Lateral Rooting Independently of the CORONATINE INSENSITIVE1 Receptor.Plant Physiol. 2018 Aug;177(4):1704-1716. doi: 10.1104/pp.18.00357. Epub 2018 Jun 22. Plant Physiol. 2018. PMID: 29934297 Free PMC article.
-
Jasmonate regulates leaf senescence and tolerance to cold stress: crosstalk with other phytohormones.J Exp Bot. 2017 Mar 1;68(6):1361-1369. doi: 10.1093/jxb/erx004. J Exp Bot. 2017. PMID: 28201612 Review.
-
JAZ repressors and the orchestration of phytohormone crosstalk.Trends Plant Sci. 2012 Jan;17(1):22-31. doi: 10.1016/j.tplants.2011.10.006. Epub 2011 Nov 21. Trends Plant Sci. 2012. PMID: 22112386 Review.
Cited by
-
Transcriptional landscapes of de novo root regeneration from detached Arabidopsis leaves revealed by time-lapse and single-cell RNA sequencing analyses.Plant Commun. 2022 Jul 11;3(4):100306. doi: 10.1016/j.xplc.2022.100306. Epub 2022 Feb 25. Plant Commun. 2022. PMID: 35605192 Free PMC article.
-
Integration of Phytomelatonin Signaling With Jasmonic Acid in Wound-induced Adventitious Root Regeneration.Adv Sci (Weinh). 2025 Mar;12(11):e2413485. doi: 10.1002/advs.202413485. Epub 2025 Jan 23. Adv Sci (Weinh). 2025. PMID: 39853644 Free PMC article.
-
RNA Sequencing Analysis and Verification of Paeonia ostii 'Fengdan' CuZn Superoxide Dismutase (PoSOD) Genes in Root Development.Plants (Basel). 2024 Jan 31;13(3):421. doi: 10.3390/plants13030421. Plants (Basel). 2024. PMID: 38337954 Free PMC article.
-
Understanding the Intricate Web of Phytohormone Signalling in Modulating Root System Architecture.Int J Mol Sci. 2021 May 24;22(11):5508. doi: 10.3390/ijms22115508. Int J Mol Sci. 2021. PMID: 34073675 Free PMC article. Review.
-
Molecular Bases for the Regulation of Adventitious Root Generation in Plants.Front Plant Sci. 2021 Jan 28;12:614072. doi: 10.3389/fpls.2021.614072. eCollection 2021. Front Plant Sci. 2021. PMID: 33584771 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

