Modifying Phosphate Toxicity in Chronic Kidney Disease
- PMID: 31505780
- PMCID: PMC6784221
- DOI: 10.3390/toxins11090522
Modifying Phosphate Toxicity in Chronic Kidney Disease
Abstract
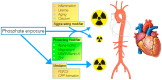
Phosphate toxicity is a well-established phenomenon, especially in chronic kidney disease (CKD), where hyperphosphatemia is a frequent occurrence when CKD is advanced. Many therapeutic efforts are targeted at phosphate, and comprise dietary intervention, modifying dialysis schemes, treating uncontrolled hyperparathyroidism and importantly, phosphate binder therapy. Despite all these interventions, hyperphosphatemia persists in many, and its pathological influence is ongoing. In nephrological care, a somewhat neglected aspect of treatment-when attempts fail to lower exposure to a toxin like phosphate-is to explore the possibility of "anti-dotes". Indeed, quite a long list of factors modify, or are mediators of phosphate toxicity. Addressing these, especially when phosphate itself cannot be sufficiently controlled, may provide additional protection. In this narrative overview, several factors are discussed that may qualify as either such a modifier or mediator, that can be influenced by other means than simply lowering phosphate exposure. A wider scope when targeting phosphate-induced comorbidity in CKD, in particular cardiovascular disease, may alleviate the burden of disease that is the consequence of this potentially toxic mineral in CKD.
Keywords: CKD-MBD; chronic kidney disease; phosphate.
Conflict of interest statement
The author reports no conflict of interest related to this manuscript.
Figures

References
-
- Vervloet M.G., Sezer S., Massy Z.A., Johansson L., Cozzolino M., Fouque D., ERA–EDTA Working Group on Chronic Kidney Disease–Mineral and Bone Disorders and the European Renal Nutrition Working Group The role of phosphate in kidney disease. Nat. Rev. Nephrol. 2016 doi: 10.1038/nrneph.2016.164. - DOI - PubMed
-
- Ketteler M., Block G.A., Evenepoel P., Fukagawa M., Herzog C.A., McCann L., Moe S.M., Shroff R., Tonelli M.A., Toussaint N.D., et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline Update: What’s changed and why it matters. Kidney Int. 2017;92:26–36. doi: 10.1016/j.kint.2017.04.006. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

