Numerical Simulation of Electroactive Hydrogels for Cartilage-Tissue Engineering
- PMID: 31505797
- PMCID: PMC6774344
- DOI: 10.3390/ma12182913
Numerical Simulation of Electroactive Hydrogels for Cartilage-Tissue Engineering
Abstract
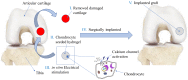
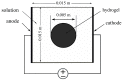
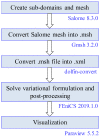
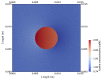
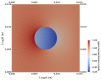
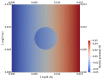
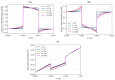
The intrinsic regeneration potential of hyaline cartilage is highly limited due to the absence of blood vessels, lymphatics, and nerves, as well as a low cell turnover within the tissue. Despite various advancements in the field of regenerative medicine, it remains a challenge to remedy articular cartilage defects resulting from trauma, aging, or osteoarthritis. Among various approaches, tissue engineering using tailored electroactive scaffolds has evolved as a promising strategy to repair damaged cartilage tissue. In this approach, hydrogel scaffolds are used as artificial extracellular matrices, and electric stimulation is applied to facilitate proliferation, differentiation, and cell growth at the defect site. In this regard, we present a simulation model of electroactive hydrogels to be used for cartilage-tissue engineering employing open-source finite-element software FEniCS together with a Python interface. The proposed mathematical formulation was first validated with an example from the literature. Then, we computed the effect of electric stimulation on a circular hydrogel sample that served as a model for a cartilage-repair implant.
Keywords: articular cartilage; cartilage–tissue engineering; computational modelling; electrical stimulation; electrically conductive hydrogels; scaffold.
Conflict of interest statement
The authors declare no conflict of interest. The authors alone are responsible for the content and writing of the paper.
Figures



Similar articles
-
Numerical Simulations as Means for Tailoring Electrically Conductive Hydrogels Towards Cartilage Tissue Engineering by Electrical Stimulation.Molecules. 2020 Oct 16;25(20):4750. doi: 10.3390/molecules25204750. Molecules. 2020. PMID: 33081205 Free PMC article.
-
Computational study on electromechanics of electroactive hydrogels for cartilage-tissue repair.Comput Methods Programs Biomed. 2020 Dec;197:105739. doi: 10.1016/j.cmpb.2020.105739. Epub 2020 Sep 12. Comput Methods Programs Biomed. 2020. PMID: 32950923
-
A phase-field model for articular cartilage regeneration in degradable scaffolds.Bull Math Biol. 2013 Dec;75(12):2389-409. doi: 10.1007/s11538-013-9897-3. Epub 2013 Sep 26. Bull Math Biol. 2013. PMID: 24072660
-
Electrically Conductive Hydrogels for Articular Cartilage Tissue Engineering.Gels. 2022 Nov 3;8(11):710. doi: 10.3390/gels8110710. Gels. 2022. PMID: 36354618 Free PMC article. Review.
-
Advancements in tissue engineering for articular cartilage regeneration.Heliyon. 2024 Feb 1;10(3):e25400. doi: 10.1016/j.heliyon.2024.e25400. eCollection 2024 Feb 15. Heliyon. 2024. PMID: 38352769 Free PMC article. Review.
Cited by
-
Numerical Simulations as Means for Tailoring Electrically Conductive Hydrogels Towards Cartilage Tissue Engineering by Electrical Stimulation.Molecules. 2020 Oct 16;25(20):4750. doi: 10.3390/molecules25204750. Molecules. 2020. PMID: 33081205 Free PMC article.
-
Tissue engineering and future directions in regenerative medicine for knee cartilage repair: a comprehensive review.Croat Med J. 2024 Jun 13;65(3):268-287. doi: 10.3325/cmj.2024.65.268. Croat Med J. 2024. PMID: 38868973 Free PMC article. Review.
-
Advanced Hydrogels for Cartilage Tissue Engineering: Recent Progress and Future Directions.Polymers (Basel). 2021 Nov 30;13(23):4199. doi: 10.3390/polym13234199. Polymers (Basel). 2021. PMID: 34883702 Free PMC article. Review.
-
"Lessons from Rare Forms of Osteoarthritis".Calcif Tissue Int. 2021 Sep;109(3):291-302. doi: 10.1007/s00223-021-00896-3. Epub 2021 Aug 21. Calcif Tissue Int. 2021. PMID: 34417863 Free PMC article. Review.
-
Using a Digital Twin of an Electrical Stimulation Device to Monitor and Control the Electrical Stimulation of Cells in vitro.Front Bioeng Biotechnol. 2021 Dec 8;9:765516. doi: 10.3389/fbioe.2021.765516. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34957068 Free PMC article.
References
-
- De Mattei M., Pellati A., Pasello M., Ongaro A., Setti S., Massari L., Gemmati D., Caruso A. Effects of physical stimulation with electromagnetic field and insulin growth factor-I treatment on proteoglycan synthesis of bovine articular cartilage. Osteoarthr. Cartil. 2004;12:793–800. doi: 10.1016/j.joca.2004.06.012. - DOI - PubMed
LinkOut - more resources
Full Text Sources

