MRI-Tracking of Dental Pulp Stem Cells In Vitro and In Vivo Using Dextran-Coated Superparamagnetic Iron Oxide Nanoparticles
- PMID: 31505807
- PMCID: PMC6780915
- DOI: 10.3390/jcm8091418
MRI-Tracking of Dental Pulp Stem Cells In Vitro and In Vivo Using Dextran-Coated Superparamagnetic Iron Oxide Nanoparticles
Abstract
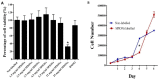
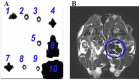
The aim of this study was to track dental pulp stem cells (DPSCs) labeled with dextran-coated superparamagnetic iron oxide nanoparticles (SPIONs) using magnetic resonance imaging (MRI). Dental pulp was isolated from male Sprague Dawley rats and cultured in Dulbecco's modified Eagle's medium F12 (DMEM-F12) and 10% fetal bovine serum. Effects of SPIONs on morphology, viability, apoptosis, stemness, and osteogenic and adipogenic differentiation of DPSCs were assessed. Prussian blue staining and MRI were conducted to determine in vitro efficiency of SPIONs uptake by the cells. Both non-labeled and labeled DPSCs were adherent to culture plates and showed spindle-shape morphologies, respectively. They were positive for osteogenic and adipogenic induction and expression of cluster of differentiation (CD) 73 and CD90 biomarkers, but negative for expression of CD34 and CD45 biomarkers. The SPIONs were non-toxic and did not induce apoptosis in doses less than 25 mg/mL. Internalization of the SPIONs within the DPSCs was confirmed by Prussian blue staining and MRI. Our findings revealed that the MRI-based method could successfully monitor DPSCs labeled with dextran-coated SPIONs without any significant effect on osteogenic and adipogenic differentiation, viability, and stemness of DPSCs. We provided the in vitro evidence supporting the feasibility of an MRI-based method to monitor DPSCs labeled with SPIONs without any significant reduction in viability, proliferation, and differentiation properties of labeled cells, showing that internalization of SPIONs within DPSCs were not toxic at doses less than 25 mg/mL. In general, the SPION labeling does not seem to impair cell survival or differentiation. SPIONs are biocompatible, easily available, and cost effective, opening a new avenue in stem cell labeling in regenerative medicine.
Keywords: MRI; dental pulp stem cells; iron oxide; labeling; nanoparticle; tracking.
Conflict of interest statement
The authors declare no conflicts of interest in this work.
Figures



Similar articles
-
Cell Proliferation, Viability, Differentiation, and Apoptosis of Iron Oxide Labeled Stem Cells Transfected with Lipofectamine Assessed by MRI.J Clin Med. 2023 Mar 20;12(6):2395. doi: 10.3390/jcm12062395. J Clin Med. 2023. PMID: 36983399 Free PMC article.
-
Highly efficient magnetic stem cell labeling with citrate-coated superparamagnetic iron oxide nanoparticles for MRI tracking.Biomaterials. 2012 Jun;33(18):4515-25. doi: 10.1016/j.biomaterials.2012.02.064. Epub 2012 Mar 24. Biomaterials. 2012. PMID: 22445482
-
Tracking the healing effect of human Wharton's jelly stem cells labeled with superparamagnetic iron oxide nanoparticles seeded onto polyvinyl alcohol/chitosan/carbon nanotubes in burn wounds by MRI and Prussian blue staining.Biomed Mater. 2025 Mar 3;20(2). doi: 10.1088/1748-605X/ad9fc6. Biomed Mater. 2025. PMID: 39681069
-
Tracking stem cells with superparamagnetic iron oxide nanoparticles: perspectives and considerations.Int J Nanomedicine. 2017 Jan 25;12:779-793. doi: 10.2147/IJN.S126530. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28182122 Free PMC article. Review.
-
Advances in superparamagnetic iron oxide nanoparticles modified with branched polyethyleneimine for multimodal imaging.Front Bioeng Biotechnol. 2024 Jan 25;11:1323316. doi: 10.3389/fbioe.2023.1323316. eCollection 2023. Front Bioeng Biotechnol. 2024. PMID: 38333548 Free PMC article. Review.
Cited by
-
Applications of superparamagnetic iron oxide nanoparticles in drug and therapeutic delivery, and biotechnological advancements.Beilstein J Nanotechnol. 2020 Jul 27;11:1092-1109. doi: 10.3762/bjnano.11.94. eCollection 2020. Beilstein J Nanotechnol. 2020. PMID: 32802712 Free PMC article. Review.
-
Proliferative and Regenerative Effect of Acetonic Extract of Feijoa sellowiana on Stem Cells.World J Plast Surg. 2020 Sep;9(3):313-320. doi: 10.29252/wjps.9.3.313. World J Plast Surg. 2020. PMID: 33330009 Free PMC article.
-
Use of Magnetic Resonance Imaging to Assess the Regenerative Effects of Adipose Tissue-Derived Mesenchymal Stem Cells in a Rabbit Cartilaginous Laryngeal Defect Model.Curr Ther Res Clin Exp. 2022 Jul 22;97:100682. doi: 10.1016/j.curtheres.2022.100682. eCollection 2022. Curr Ther Res Clin Exp. 2022. PMID: 35959231 Free PMC article.
-
In Vivo Stem Cell Imaging Principles and Applications.Int J Stem Cells. 2023 Nov 30;16(4):363-375. doi: 10.15283/ijsc23045. Epub 2023 Aug 30. Int J Stem Cells. 2023. PMID: 37643761 Free PMC article. Review.
-
The impact of acemannan, an extracted product from Aloe vera, on proliferation of dental pulp stem cells and healing of mandibular defects in rabbits.Am J Stem Cells. 2024 Apr 25;13(2):75-86. doi: 10.62347/UAFC3719. eCollection 2024. Am J Stem Cells. 2024. PMID: 38765804 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

