The Intestine of Drosophila melanogaster: An Emerging Versatile Model System to Study Intestinal Epithelial Homeostasis and Host-Microbial Interactions in Humans
- PMID: 31505811
- PMCID: PMC6780840
- DOI: 10.3390/microorganisms7090336
The Intestine of Drosophila melanogaster: An Emerging Versatile Model System to Study Intestinal Epithelial Homeostasis and Host-Microbial Interactions in Humans
Abstract
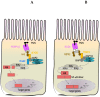
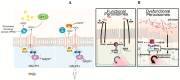
In all metazoans, the intestinal tract is an essential organ to integrate nutritional signaling, hormonal cues and immunometabolic networks. The dysregulation of intestinal epithelium functions can impact organism physiology and, in humans, leads to devastating and complex diseases, such as inflammatory bowel diseases, intestinal cancers, and obesity. Two decades ago, the discovery of an immune response in the intestine of the genetic model system, Drosophila melanogaster, sparked interest in using this model organism to dissect the mechanisms that govern gut (patho) physiology in humans. In 2007, the finding of the intestinal stem cell lineage, followed by the development of tools available for its manipulation in vivo, helped to elucidate the structural organization and functions of the fly intestine and its similarity with mammalian gastrointestinal systems. To date, studies of the Drosophila gut have already helped to shed light on a broad range of biological questions regarding stem cells and their niches, interorgan communication, immunity and immunometabolism, making the Drosophila a promising model organism for human enteric studies. This review summarizes our current knowledge of the structure and functions of the Drosophila melanogaster intestine, asserting its validity as an emerging model system to study gut physiology, regeneration, immune defenses and host-microbiota interactions.
Keywords: Drosophila melanogaster; host-pathogen/commensal interactions; immunometabolism; inflammatory bowel disease; innate immunity; intestinal epithelium; microbiota; midgut; small intestine.
Conflict of interest statement
The authors declare no conflict of interests.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

