Temozolomide and Other Alkylating Agents in Glioblastoma Therapy
- PMID: 31505812
- PMCID: PMC6783999
- DOI: 10.3390/biomedicines7030069
Temozolomide and Other Alkylating Agents in Glioblastoma Therapy
Abstract
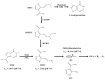
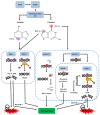
The alkylating agent temozolomide (TMZ) together with maximal safe bulk resection and focal radiotherapy comprises the standard treatment for glioblastoma (GB), a particularly aggressive and lethal primary brain tumor. GB affects 3.2 in 100,000 people who have an average survival time of around 14 months after presentation. Several key aspects make GB a difficult to treat disease, primarily including the high resistance of tumor cells to cell death-inducing substances or radiation and the combination of the highly invasive nature of the malignancy, i.e., treatment must affect the whole brain, and the protection from drugs of the tumor bulk-or at least of the invading cells-by the blood brain barrier (BBB). TMZ crosses the BBB, but-unlike classic chemotherapeutics-does not induce DNA damage or misalignment of segregating chromosomes directly. It has been described as a DNA alkylating agent, which leads to base mismatches that initiate futile DNA repair cycles; eventually, DNA strand breaks, which in turn induces cell death. However, while much is assumed about the function of TMZ and its mode of action, primary data are actually scarce and often contradictory. To improve GB treatment further, we need to fully understand what TMZ does to the tumor cells and their microenvironment. This is of particular importance, as novel therapeutic approaches are almost always clinically assessed in the presence of standard treatment, i.e., in the presence of TMZ. Therefore, potential pharmacological interactions between TMZ and novel drugs might occur with unforeseeable consequences.
Keywords: alkylating agents; brain tumor; glioblastoma; temozolomide (TMZ), triazene compounds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Louis D.N., Perry A., Reifenberger G., Deimling A., von Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. - DOI - PubMed
-
- Stupp R., Hegi M.E., Mason W.P., van den Bent M.J., Taphoorn M.J.B., Janzer R.C., Ludwin S.K., Allgeier A., Fisher B., Belanger K., et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

