Regulation of Ascorbate-Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress
- PMID: 31505852
- PMCID: PMC6770940
- DOI: 10.3390/antiox8090384
Regulation of Ascorbate-Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress
Abstract
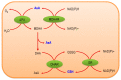
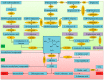
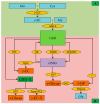
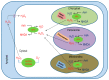
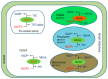
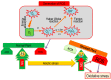
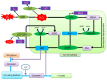
Reactive oxygen species (ROS) generation is a usual phenomenon in a plant both under a normal and stressed condition. However, under unfavorable or adverse conditions, ROS production exceeds the capacity of the antioxidant defense system. Both non-enzymatic and enzymatic components of the antioxidant defense system either detoxify or scavenge ROS and mitigate their deleterious effects. The Ascorbate-Glutathione (AsA-GSH) pathway, also known as Asada-Halliwell pathway comprises of AsA, GSH, and four enzymes viz. ascorbate peroxidase, monodehydroascorbate reductase, dehydroascorbate reductase, and glutathione reductase, play a vital role in detoxifying ROS. Apart from ROS detoxification, they also interact with other defense systems in plants and protect the plants from various abiotic stress-induced damages. Several plant studies revealed that the upregulation or overexpression of AsA-GSH pathway enzymes and the enhancement of the AsA and GSH levels conferred plants better tolerance to abiotic stresses by reducing the ROS. In this review, we summarize the recent progress of the research on AsA-GSH pathway in terms of oxidative stress tolerance in plants. We also focus on the defense mechanisms as well as molecular interactions.
Keywords: antioxidant defense; free radicals; glyoxalase system; hydrogen peroxide; plant abiotic stress; reactive oxygen species; redox biology; stress signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Coordinated Actions of Glyoxalase and Antioxidant Defense Systems in Conferring Abiotic Stress Tolerance in Plants.Int J Mol Sci. 2017 Jan 20;18(1):200. doi: 10.3390/ijms18010200. Int J Mol Sci. 2017. PMID: 28117669 Free PMC article. Review.
-
Hydrogen Peroxide Pretreatment Mitigates Cadmium-Induced Oxidative Stress in Brassica napus L.: An Intrinsic Study on Antioxidant Defense and Glyoxalase Systems.Front Plant Sci. 2017 Feb 10;8:115. doi: 10.3389/fpls.2017.00115. eCollection 2017. Front Plant Sci. 2017. PMID: 28239385 Free PMC article.
-
Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants.Plant Physiol Biochem. 2010 Dec;48(12):909-30. doi: 10.1016/j.plaphy.2010.08.016. Epub 2010 Sep 15. Plant Physiol Biochem. 2010. PMID: 20870416 Review.
-
Glutathione and glutathione reductase: a boon in disguise for plant abiotic stress defense operations.Plant Physiol Biochem. 2013 Sep;70:204-12. doi: 10.1016/j.plaphy.2013.05.032. Epub 2013 Jun 3. Plant Physiol Biochem. 2013. PMID: 23792825 Review.
-
Micronutrient fertilization enhances ROS scavenging system for alleviation of abiotic stresses in plants.Plant Physiol Biochem. 2021 Mar;160:386-396. doi: 10.1016/j.plaphy.2021.01.040. Epub 2021 Jan 30. Plant Physiol Biochem. 2021. PMID: 33556754 Review.
Cited by
-
Tebuconazole and trifloxystrobin regulate the physiology, antioxidant defense and methylglyoxal detoxification systems in conferring salt stress tolerance in Triticum aestivum L.Physiol Mol Biol Plants. 2020 Jun;26(6):1139-1154. doi: 10.1007/s12298-020-00810-5. Epub 2020 May 15. Physiol Mol Biol Plants. 2020. PMID: 32549679 Free PMC article.
-
Integrative Analysis of Transcriptome and Metabolome Reveals Salt Stress Orchestrating the Accumulation of Specialized Metabolites in Lycium barbarum L. Fruit.Int J Mol Sci. 2021 Apr 23;22(9):4414. doi: 10.3390/ijms22094414. Int J Mol Sci. 2021. PMID: 33922536 Free PMC article.
-
Biochemical Insights into the Ability of Lemna minor L. Extract to Counteract Copper Toxicity in Maize.Plants (Basel). 2022 Oct 6;11(19):2613. doi: 10.3390/plants11192613. Plants (Basel). 2022. PMID: 36235490 Free PMC article.
-
Glutathione metabolism in Cryptocaryon irritans involved in defense against oxidative stress induced by zinc ions.Parasit Vectors. 2022 Sep 7;15(1):318. doi: 10.1186/s13071-022-05390-9. Parasit Vectors. 2022. PMID: 36071467 Free PMC article.
-
Cobalt and Titanium Alleviate the Methylglyoxal-Induced Oxidative Stress in Pennisetum divisum Seedlings under Saline Conditions.Metabolites. 2023 Nov 19;13(11):1162. doi: 10.3390/metabo13111162. Metabolites. 2023. PMID: 37999257 Free PMC article.
References
-
- Hasanuzzaman M., Mahmud J.A., Nahar K., Anee T.I., Inafuku M., Oku H., Fujita M. Responses, adaptation, and ROS metabolism in plants exposed to waterlogging stress. In: Khan M.I.R., Khan N.A., editors. Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation under Abiotic Stress. Springer; New York, NY, USA: 2017.
-
- Halliwell B., Gutteridge J.M.C. Antioxidant defences: Endogenous and diet derived. Free Radic. Biol. Med. 2007;4:79–186.
Publication types
LinkOut - more resources
Full Text Sources

