XIAP as a Target of New Small Organic Natural Molecules Inducing Human Cancer Cell Death
- PMID: 31505859
- PMCID: PMC6770071
- DOI: 10.3390/cancers11091336
XIAP as a Target of New Small Organic Natural Molecules Inducing Human Cancer Cell Death
Abstract
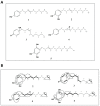
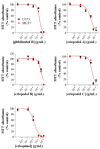
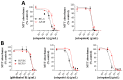
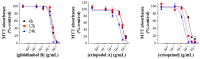
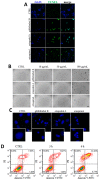
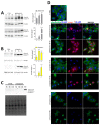
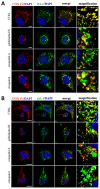
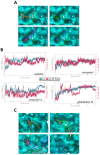
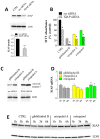
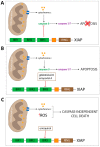
X-linked inhibitor of apoptosis protein (XIAP) is an emerging crucial therapeutic target in cancer. We report on the discovery and characterisation of small organic molecules from Piper genus plants exhibiting XIAP antagonism, namely erioquinol, a quinol substituted in the 4-position with an alkenyl group and the alkenylphenols eriopodols A-C. Another isolated compound was originally identified as gibbilimbol B. Erioquinol was the most potent inhibitor of human cancer cell viability when compared with gibbilimbol B and eriopodol A was listed as intermediate. Gibbilimbol B and eriopodol A induced apoptosis through mitochondrial permeabilisation and caspase activation while erioquinol acted on cell fate via caspase-independent/non-apoptotic mechanisms, likely involving mitochondrial dysfunctions and aberrant generation of reactive oxygen species. In silico modelling and molecular approaches suggested that all molecules inhibit XIAP by binding to XIAP-baculoviral IAP repeat domain. This demonstrates a novel aspect of XIAP as a key determinant of tumour control, at the molecular crossroad of caspase-dependent/independent cell death pathway and indicates molecular aspects to develop tumour-effective XIAP antagonists.
Keywords: Piper eriopodon, alkenylphenols; XIAP antagonists; XIAP-BIR3 domain; apoptosis; caspase-independent cell death; cell death; human cancer cells; phytochemicals; small organic agents.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

