Twenty Years of Ferroportin Disease: A Review or An Update of Published Clinical, Biochemical, Molecular, and Functional Features
- PMID: 31505869
- PMCID: PMC6789780
- DOI: 10.3390/ph12030132
Twenty Years of Ferroportin Disease: A Review or An Update of Published Clinical, Biochemical, Molecular, and Functional Features
Abstract
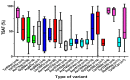
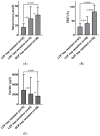
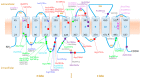
Iron overloading disorders linked to mutations in ferroportin have diverse phenotypes in vivo, and the effects of mutations on ferroportin in vitro range from loss of function (LOF) to gain of function (GOF) with hepcidin resistance. We reviewed 359 patients with 60 ferroportin variants. Overall, macrophage iron overload and low/normal transferrin saturation (TSAT) segregated with mutations that caused LOF, while GOF mutations were linked to high TSAT and parenchymal iron accumulation. However, the pathogenicity of individual variants is difficult to establish due to the lack of sufficiently reported data, large inter-assay variability of functional studies, and the uncertainty associated with the performance of available in silico prediction models. Since the phenotypes of hepcidin-resistant GOF variants are indistinguishable from the other types of hereditary hemochromatosis (HH), these variants may be categorized as ferroportin-associated HH, while the entity ferroportin disease may be confined to patients with LOF variants. To further improve the management of ferroportin disease, we advocate for a global registry, with standardized clinical analysis and validation of the functional tests preferably performed in human-derived enterocytic and macrophagic cell lines. Moreover, studies are warranted to unravel the definite structure of ferroportin and the indispensable residues that are essential for functionality.
Keywords: SLC40A1; ferritin; ferroportin; hemochromatosis; iron overload; non-HFE.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The Spectra of Disease-Causing Mutations in the Ferroportin 1 (SLC40A1) Encoding Gene and Related Iron Overload Phenotypes (Hemochromatosis Type 4 and Ferroportin Disease).Hum Mutat. 2023 Jun 13;2023:5162256. doi: 10.1155/2023/5162256. eCollection 2023. Hum Mutat. 2023. PMID: 40225168 Free PMC article. Review.
-
Inherited Disorders of Iron Overload.Front Nutr. 2018 Oct 29;5:103. doi: 10.3389/fnut.2018.00103. eCollection 2018. Front Nutr. 2018. PMID: 30420953 Free PMC article. Review.
-
Inherited iron overload disorders.Transl Gastroenterol Hepatol. 2020 Apr 5;5:25. doi: 10.21037/tgh.2019.11.15. eCollection 2020. Transl Gastroenterol Hepatol. 2020. PMID: 32258529 Free PMC article. Review.
-
Reduced iron export associated with hepcidin resistance can explain the iron overload spectrum in ferroportin disease.Liver Int. 2020 Aug;40(8):1941-1951. doi: 10.1111/liv.14539. Epub 2020 Jun 12. Liver Int. 2020. PMID: 32450003 Free PMC article.
-
Impact of D181V and A69T on the function of ferroportin as an iron export pump and hepcidin receptor.Biochim Biophys Acta. 2014 Sep;1842(9):1406-12. doi: 10.1016/j.bbadis.2014.05.011. Epub 2014 May 21. Biochim Biophys Acta. 2014. PMID: 24859227
Cited by
-
Genetic Incorporation of Dansylalanine in Human Ferroportin to Probe the Alternating Access Mechanism of Iron Transport.Int J Mol Sci. 2023 Jul 25;24(15):11919. doi: 10.3390/ijms241511919. Int J Mol Sci. 2023. PMID: 37569293 Free PMC article.
-
Identification of Novel Mutations by Targeted NGS Panel in Patients with Hyperferritinemia.Genes (Basel). 2021 Nov 9;12(11):1778. doi: 10.3390/genes12111778. Genes (Basel). 2021. PMID: 34828384 Free PMC article.
-
Iron as Therapeutic Target in Human Diseases.Pharmaceuticals (Basel). 2019 Dec 5;12(4):178. doi: 10.3390/ph12040178. Pharmaceuticals (Basel). 2019. PMID: 31817314 Free PMC article.
-
Hemochromatosis classification: update and recommendations by the BIOIRON Society.Blood. 2022 May 19;139(20):3018-3029. doi: 10.1182/blood.2021011338. Blood. 2022. PMID: 34601591 Free PMC article.
-
The iron chaperone poly(rC)-binding protein 1 regulates iron efflux through intestinal ferroportin in mice.Blood. 2023 Nov 9;142(19):1658-1671. doi: 10.1182/blood.2023020504. Blood. 2023. PMID: 37624904 Free PMC article.
References
-
- Pietrangelo A., Montosi G., Totaro A., Garuti C., Fraquelli M., Sardini C., Vasta F., Pietrangelo A., Conte D., Cassanelli S., et al. Hereditary hemochromatosis in adults without pathogenic mutations in the hemochromatosis gene. N. Engl. J. Med. 1999;341:725–732. doi: 10.1056/NEJM199909023411003. - DOI - PubMed
-
- Montosi G., Donovan A., Totaro A., Garuti C., Pignatti E., Cassanelli S., Trenor C.C., Gasparini P., Andrews N.C., Pietrangelo A. Autosomal-dominant hemochromatosis is associated with a mutation in the ferroportin (SLC11A3) gene. J. Clin. Investig. 2001;108:619–623. doi: 10.1172/JCI200113468. - DOI - PMC - PubMed
-
- Nemeth E. Ferroportin mutations: A tale of two phenotypes. Blood. 2005;105:3763–3764. doi: 10.1182/blood-2005-02-0771. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

