Antioxidant Activity of Selected Stilbenoid Derivatives in a Cellular Model System
- PMID: 31505897
- PMCID: PMC6770161
- DOI: 10.3390/biom9090468
Antioxidant Activity of Selected Stilbenoid Derivatives in a Cellular Model System
Abstract
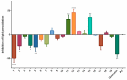
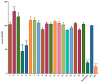
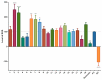
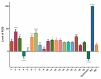
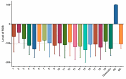
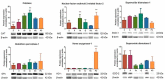
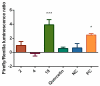
The stilbenoids, a group of naturally occurring phenolic compounds, are found in a variety of plants, including some berries that are used as food or for medicinal purposes. They are known to be beneficial for human health as anti-inflammatory, chemopreventive, and antioxidative agents. We have investigated a group of 19 stilbenoid substances in vitro using a cellular model of THP-1 macrophage-like cells and pyocyanin-induced oxidative stress to evaluate their antioxidant or pro-oxidant properties. Then we have determined any effects that they might have on the expression of the enzymes catalase, glutathione peroxidase, and heme oxygenase-1, and their effects on the activation of Nrf2. The experimental results showed that these stilbenoids could affect the formation of reactive oxygen species in a cellular model, producing either an antioxidative or pro-oxidative effect, depending on the structure pinostilbene (2) worked as a pro-oxidant and also decreased expression of catalase in the cell culture. Piceatannol (4) had shown reactive oxygen species (ROS) scavenging activity, whereas isorhapontigenin (18) had a mild direct antioxidant effect and activated Nrf2-antioxidant response element (ARE) system and elevated expression of Nrf2 and catalase. Their effects shown on cells in vitro warrant their further study in vivo.
Keywords: Nrf2; antioxidant; macrophages; pro-oxidant; pyocyanin; stilbenoid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The berry constituents quercetin, kaempferol, and pterostilbene synergistically attenuate reactive oxygen species: involvement of the Nrf2-ARE signaling pathway.Food Chem Toxicol. 2014 Oct;72:303-11. doi: 10.1016/j.fct.2014.07.038. Epub 2014 Aug 8. Food Chem Toxicol. 2014. PMID: 25111660
-
Piceatannol induces heme oxygenase-1 expression in human mammary epithelial cells through activation of ARE-driven Nrf2 signaling.Arch Biochem Biophys. 2010 Sep 1;501(1):142-50. doi: 10.1016/j.abb.2010.06.011. Epub 2010 Jun 15. Arch Biochem Biophys. 2010. PMID: 20558128
-
Isoflavone biochanin A, a novel nuclear factor erythroid 2-related factor 2 (Nrf2)-antioxidant response element activator, protects against oxidative damage in HepG2 cells.Biofactors. 2019 Jul;45(4):563-574. doi: 10.1002/biof.1514. Epub 2019 May 27. Biofactors. 2019. PMID: 31131946
-
Role of resveratrol in regulation of cellular defense systems against oxidative stress.Biofactors. 2018 Jan;44(1):36-49. doi: 10.1002/biof.1399. Epub 2017 Nov 28. Biofactors. 2018. PMID: 29193412 Review.
-
Resveratrol as an antioxidant and pro-oxidant agent: mechanisms and clinical implications.Biochem Soc Trans. 2007 Nov;35(Pt 5):1156-60. doi: 10.1042/BST0351156. Biochem Soc Trans. 2007. PMID: 17956300 Review.
Cited by
-
Direct and Indirect Antioxidant Effects of Selected Plant Phenolics in Cell-Based Assays.Molecules. 2021 Apr 26;26(9):2534. doi: 10.3390/molecules26092534. Molecules. 2021. PMID: 33926137 Free PMC article.
-
Potential Association of Reactive Oxygen Species With Male Sterility in Peach.Front Plant Sci. 2021 Apr 14;12:653256. doi: 10.3389/fpls.2021.653256. eCollection 2021. Front Plant Sci. 2021. PMID: 33936139 Free PMC article.
-
Treating Cancers Using Nature's Medicine: Significance and Challenges.Biomolecules. 2021 Nov 15;11(11):1698. doi: 10.3390/biom11111698. Biomolecules. 2021. PMID: 34827696 Free PMC article.
-
Antioxidative Activity of 1,3,5-Triazine Analogues Incorporating Aminobenzene Sulfonamide, Aminoalcohol/Phenol, Piperazine, Chalcone, or Stilbene Motifs.Molecules. 2020 Apr 14;25(8):1787. doi: 10.3390/molecules25081787. Molecules. 2020. PMID: 32295147 Free PMC article.
-
Identification of Plant Phenolics from Paulownia tomentosa and Morus alba as Novel PPARγ Partial Agonists and Hypoglycemic Agents.J Agric Food Chem. 2025 Jun 4;73(22):13960-13972. doi: 10.1021/acs.jafc.4c11398. Epub 2025 May 20. J Agric Food Chem. 2025. PMID: 40392982 Free PMC article.
References
-
- Leláková V., Šmejkal K., Jakubczyk K., Veselý O., Landa P., Václavík J., Bobáľ P., Pížová H., Temml V., Steinacher T., et al. Parallel in vitro and in silico investigations into anti-inflammatory effects of non-prenylated stilbenoids. Food Chem. 2019;285:431–440. doi: 10.1016/j.foodchem.2019.01.128. - DOI - PubMed
-
- Hall S., McDermott C., Anoopkumar-Dukie S., McFarland A.J., Forbes A., Perkins A.V., Davey A.K., Chess-Williams R., Kiefel M.J., Arora D., et al. Cellular Effects of Pyocyanin, a Secreted Virulence Factor of Pseudomonas aeruginosa. Toxins. 2016;8:236. doi: 10.3390/toxins8080236. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

