Ten-Day Concomitant, 10-Day Sequential, and 7-Day Triple Therapy as First-Line Treatment for Helicobacter pylori Infection: A Nationwide Randomized Trial in Korea
- PMID: 31505907
- PMCID: PMC6743805
- DOI: 10.5009/gnl19136
Ten-Day Concomitant, 10-Day Sequential, and 7-Day Triple Therapy as First-Line Treatment for Helicobacter pylori Infection: A Nationwide Randomized Trial in Korea
Abstract
Background/aims: This nationwide, multicenter prospective randomized controlled trial aimed to compare the efficacy and safety of 10-day concomitant therapy (CT) and 10-day sequential therapy (ST) with 7-day clarithromycin-containing triple therapy (TT) as first-line treatment for Helicobacter pylori infection in the Korean population.
Methods: Patients with H. pylori infection were assigned randomly to 7d-TT (lansoprazole 30 mg, amoxicillin 1 g, and clarithromycin 500 mg twice daily for 7 days), 10d-ST (lansoprazole 30 mg and amoxicillin 1 g twice daily for the first 5 days, followed by lansoprazole 30 mg, clarithromycin 500 mg, and metronidazole 500 mg twice daily for the remaining 5 days), or 10d-CT (lansoprazole 30 mg, amoxicillin 1 g, clarithromycin 500 mg, and metronidazole 500 mg twice daily for 10 days). The primary endpoint was eradication rate by intention-to-treat (ITT) and per-protocol (PP) analyses.
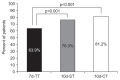
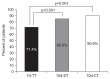
Results: A total of 1,141 patients were included. The 10d-CT protocol achieved a markedly higher eradication rate than the 7d-TT protocol in both the ITT (81.2% vs 63.9%) and PP analyses (90.6% vs 71.4%). The eradication rate of the 10d-ST protocol was superior to that of the 7d-TT protocol (76.3% vs 63.9%, ITT analysis; 85.0% vs 71.4%, PP analysis). No significant differences in adherence or serious side effects were found among the three treatment arms.
Conclusions: The 10d-CT and 10d-ST regimens were superior to the 7d-TT regimen as standard first-line treatment in Korea.
Keywords: Concomitant therapy; Disease eradication; Helicobacter pylori; Sequential therapy; Triple therapy.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
Similar articles
-
Ten-day high-dose proton pump inhibitor triple therapy versus sequential therapy for Helicobacter pylori eradication.J Gastroenterol Hepatol. 2018 Nov;33(11):1822-1828. doi: 10.1111/jgh.14292. Epub 2018 Jun 27. J Gastroenterol Hepatol. 2018. PMID: 29804294 Clinical Trial.
-
Concomitant, sequential, and 7-day triple therapy in first-line treatment of Helicobacter pylori infection in Korea: study protocol for a randomized controlled trial.Trials. 2017 Nov 17;18(1):549. doi: 10.1186/s13063-017-2281-0. Trials. 2017. PMID: 29149904 Free PMC article. Clinical Trial.
-
14-day triple, 5-day concomitant, and 10-day sequential therapies for Helicobacter pylori infection in seven Latin American sites: a randomised trial.Lancet. 2011 Aug 6;378(9790):507-14. doi: 10.1016/S0140-6736(11)60825-8. Epub 2011 Jul 21. Lancet. 2011. PMID: 21777974 Free PMC article. Clinical Trial.
-
Standard triple therapy in Helicobacter pylori eradication in Turkey: Systematic evaluation and meta-analysis of 10-year studies.Turk J Gastroenterol. 2019 May;30(5):420-435. doi: 10.5152/tjg.2019.18693. Turk J Gastroenterol. 2019. PMID: 31060997 Free PMC article.
-
Systematic Review with Meta-Analysis: Concomitant Therapy vs. Triple Therapy for the First-Line Treatment of Helicobacter pylori Infection.Am J Gastroenterol. 2018 Oct;113(10):1444-1457. doi: 10.1038/s41395-018-0217-2. Epub 2018 Aug 31. Am J Gastroenterol. 2018. PMID: 30171216
Cited by
-
Biomaterials for Helicobacter pylori therapy: therapeutic potential and future perspectives.Gut Microbes. 2022 Jan-Dec;14(1):2120747. doi: 10.1080/19490976.2022.2120747. Gut Microbes. 2022. PMID: 36070564 Free PMC article. Review.
-
Current guidelines for Helicobacter pylori treatment in East Asia 2022: Differences among China, Japan, and South Korea.World J Clin Cases. 2022 Jul 6;10(19):6349-6359. doi: 10.12998/wjcc.v10.i19.6349. World J Clin Cases. 2022. PMID: 35979311 Free PMC article. Review.
-
Ten-day bismuth-containing quadruple therapy versus 7-day proton pump inhibitor-clarithromycin containing triple therapy as first-line empirical therapy for the Helicobacter pylori infection in Korea: a randomized open-label trial.BMC Gastroenterol. 2021 Mar 2;21(1):95. doi: 10.1186/s12876-021-01680-1. BMC Gastroenterol. 2021. PMID: 33653284 Free PMC article. Clinical Trial.
-
[Can Sequential Therapy Replace Standard Triple Therapy as Helicobacter pylori Eradication?].Korean J Helicobacter Up Gastrointest Res. 2023 Dec;23(4):235-236. doi: 10.7704/kjhugr.2023.0047. Epub 2023 Dec 8. Korean J Helicobacter Up Gastrointest Res. 2023. PMID: 40503503 Free PMC article. Korean. No abstract available.
-
Ten-day tegoprazan-based concomitant therapy as a first-line treatment for Helicobacter pylori eradication.Korean J Intern Med. 2023 Jul;38(4):493-503. doi: 10.3904/kjim.2022.345. Epub 2023 Jun 28. Korean J Intern Med. 2023. PMID: 37369525 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

