Robust synchronization of the cell cycle and the circadian clock through bidirectional coupling
- PMID: 31506042
- PMCID: PMC6769306
- DOI: 10.1098/rsif.2019.0376
Robust synchronization of the cell cycle and the circadian clock through bidirectional coupling
Abstract
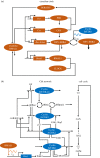
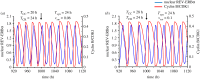
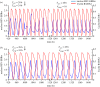
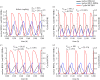
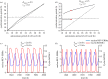
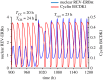
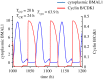
The cell cycle and the circadian clock represent major cellular rhythms, which appear to be coupled. Thus the circadian factor BMAL1 controls the level of cell cycle proteins such as Cyclin E and WEE1, the latter of which inhibits the kinase CDK1 that governs the G2/M transition. In reverse the cell cycle impinges on the circadian clock through direct control by CDK1 of REV-ERBα, which negatively regulates BMAL1. These observations provide evidence for bidirectional coupling of the cell cycle and the circadian clock. By merging detailed models for the two networks in mammalian cells, we previously showed that unidirectional coupling to the circadian clock can entrain the cell cycle to 24 or 48 h, depending on the cell cycle autonomous period, while complex oscillations occur when entrainment fails. Here we show that the reverse unidirectional coupling via phosphorylation of REV-ERBα or via mitotic inhibition of transcription, both controlled by CDK1, can elicit entrainment of the circadian clock by the cell cycle. We then determine the effect of bidirectional coupling of the cell cycle and circadian clock as a function of their relative coupling strengths. In contrast to unidirectional coupling, bidirectional coupling markedly reduces the likelihood of complex oscillations. While the two rhythms oscillate independently as long as both couplings are weak, one rhythm entrains the other if one of the couplings dominates. If the couplings in both directions become stronger and of comparable magnitude, the two rhythms synchronize, generally at an intermediate period within the range defined by the two autonomous periods prior to coupling. More surprisingly, synchronization may also occur at a period slightly below or above this range, while in some conditions the synchronization period can even be much longer. Two or even three modes of synchronization may sometimes coexist, yielding examples of birhythmicity or trirhythmicity. Because synchronization readily occurs in the form of simple periodic oscillations over a wide range of coupling strengths and in the presence of multiple connections between the two oscillatory networks, the results indicate that bidirectional coupling favours the robust synchronization of the cell cycle and the circadian clock.
Keywords: cell cycle; circadian clock; coupled oscillators; synchronization.
Conflict of interest statement
We declare we have no competing interests.
Figures
Similar articles
-
Entrainment of the mammalian cell cycle by the circadian clock: modeling two coupled cellular rhythms.PLoS Comput Biol. 2012 May;8(5):e1002516. doi: 10.1371/journal.pcbi.1002516. Epub 2012 May 31. PLoS Comput Biol. 2012. PMID: 22693436 Free PMC article.
-
Multi-rhythmicity generated by coupling two cellular rhythms.J R Soc Interface. 2019 Mar 29;16(152):20180835. doi: 10.1098/rsif.2018.0835. J R Soc Interface. 2019. PMID: 30836895 Free PMC article.
-
From circadian clock mechanism to sleep disorders and jet lag: Insights from a computational approach.Biochem Pharmacol. 2021 Sep;191:114482. doi: 10.1016/j.bcp.2021.114482. Epub 2021 Feb 20. Biochem Pharmacol. 2021. PMID: 33617843 Review.
-
Control of synchronization ratios in clock/cell cycle coupling by growth factors and glucocorticoids.R Soc Open Sci. 2020 Feb 12;7(2):192054. doi: 10.1098/rsos.192054. eCollection 2020 Feb. R Soc Open Sci. 2020. PMID: 32257354 Free PMC article.
-
WNT Takes Two to Tango: Molecular Links between the Circadian Clock and the Cell Cycle in Adult Stem Cells.J Biol Rhythms. 2018 Feb;33(1):5-14. doi: 10.1177/0748730417745913. Epub 2017 Dec 26. J Biol Rhythms. 2018. PMID: 29277155 Free PMC article. Review.
Cited by
-
A coupled model between circadian, cell-cycle, and redox rhythms reveals their regulation of oxidative stress.Sci Rep. 2024 Jul 5;14(1):15479. doi: 10.1038/s41598-024-66347-9. Sci Rep. 2024. PMID: 38969743 Free PMC article.
-
PER2 integrates circadian disruption and pituitary tumorigenesis.Theranostics. 2023 Apr 29;13(8):2657-2672. doi: 10.7150/thno.82995. eCollection 2023. Theranostics. 2023. PMID: 37215573 Free PMC article.
-
Principles, mechanisms and functions of entrainment in biological oscillators.Interface Focus. 2022 Apr 15;12(3):20210088. doi: 10.1098/rsfs.2021.0088. eCollection 2022 Jun 6. Interface Focus. 2022. PMID: 35450280 Free PMC article. Review.
-
Time of day as a critical variable in biology.BMC Biol. 2022 Jun 15;20(1):142. doi: 10.1186/s12915-022-01333-z. BMC Biol. 2022. PMID: 35705939 Free PMC article. Review.
-
Reflections on Several Landmark Advances in Circadian Biology.J Circadian Rhythms. 2024 Apr 1;22:1. doi: 10.5334/jcr.236. eCollection 2024. J Circadian Rhythms. 2024. PMID: 38617711 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

