Transcriptome analysis of the effect of C-C chemokine receptor 5 deficiency on cell response to Toxoplasma gondii in brain cells
- PMID: 31506064
- PMCID: PMC6737708
- DOI: 10.1186/s12864-019-6076-4
Transcriptome analysis of the effect of C-C chemokine receptor 5 deficiency on cell response to Toxoplasma gondii in brain cells
Abstract
Background: Infection with Toxoplasma gondii is thought to damage the brain and be a risk factor for neurological and psychotic disorders. The immune response-participating chemokine system has recently been considered vital for brain cell signaling and neural functioning. Here, we investigated the effect of the deficiency of C-C chemokine receptor 5 (CCR5), which is previously reported to be associated with T. gondii infection, on gene expression in the brain during T. gondii infection and the relationship between CCR5 and the inflammatory response against T. gondii infection in the brain.
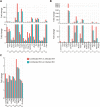
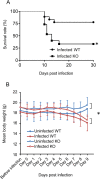
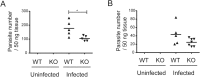
Results: We performed a genome-wide comprehensive analysis of brain cells from wild-type and CCR5-deficient mice. Mouse primary brain cells infected with T. gondii were subjected to RNA sequencing. The expression levels of some genes, especially in astrocytes and microglia, were altered by CCR5-deficiency during T. gondii infection, and the gene ontology and Kyoto Encyclopedia of Genes and Genomes analysis revealed an enhanced immune response in the brain cells. The expression levels of genes which were highly differentially expressed in vitro were also investigated in the mouse brains during the T. gondii infections. Among the genes tested, only Saa3 (serum amyloid A3) showed partly CCR5-dependent upregulation during the acute infection phase. However, analysis of the subacute phase showed that in addition to Saa3, Hmox1 may also contribute to the protection and/or pathology partly via the CCR5 pathway.
Conclusions: Our results indicate that CCR5 is involved in T. gondii infection in the brain where it contributes to inflammatory responses and parasite elimination. We suggest that the inflammatory response by glial cells through CCR5 might be associated with neurological injury during T. gondii infection to some extent.
Keywords: Astrocyte; Brain; C-C chemokine receptor 5; Microglia; Neuron; Toxoplasma gondii; Transcriptome.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Transcriptomic Analysis of the Effects of Chemokine Receptor CXCR3 Deficiency on Immune Responses in the Mouse Brain during Toxoplasma gondii Infection.Microorganisms. 2021 Nov 12;9(11):2340. doi: 10.3390/microorganisms9112340. Microorganisms. 2021. PMID: 34835465 Free PMC article.
-
Transcriptional profiling of Toll-like receptor 2-deficient primary murine brain cells during Toxoplasma gondii infection.PLoS One. 2017 Nov 14;12(11):e0187703. doi: 10.1371/journal.pone.0187703. eCollection 2017. PLoS One. 2017. PMID: 29136637 Free PMC article.
-
Cytokine responses induced by Toxoplasma gondii in astrocytes and microglial cells.Eur J Immunol. 1997 Jun;27(6):1539-48. doi: 10.1002/eji.1830270633. Eur J Immunol. 1997. PMID: 9209508
-
Persistence of Toxoplasma gondii in the central nervous system: a fine-tuned balance between the parasite, the brain and the immune system.Parasite Immunol. 2015 Mar;37(3):150-8. doi: 10.1111/pim.12173. Parasite Immunol. 2015. PMID: 25573476 Review.
-
From cells to signaling cascades: manipulation of innate immunity by Toxoplasma gondii.FEMS Immunol Med Microbiol. 2003 Dec 5;39(3):193-203. doi: 10.1016/S0928-8244(03)00279-7. FEMS Immunol Med Microbiol. 2003. PMID: 14642303 Review.
Cited by
-
Transcriptomic Analysis of the Effects of Chemokine Receptor CXCR3 Deficiency on Immune Responses in the Mouse Brain during Toxoplasma gondii Infection.Microorganisms. 2021 Nov 12;9(11):2340. doi: 10.3390/microorganisms9112340. Microorganisms. 2021. PMID: 34835465 Free PMC article.
-
Unveiling of brain transcriptome of masked palm civet (Paguma larvata) with chronic infection of Toxoplasma gondii.Parasit Vectors. 2022 Jul 24;15(1):263. doi: 10.1186/s13071-022-05378-5. Parasit Vectors. 2022. PMID: 35871661 Free PMC article.
-
Functional characterization of acyl-CoA binding protein in Neospora caninum.Parasit Vectors. 2020 Feb 18;13(1):85. doi: 10.1186/s13071-020-3967-9. Parasit Vectors. 2020. PMID: 32070415 Free PMC article.
-
The role of IFN-γ-mediated host immune responses in monitoring and the elimination of Toxoplasma gondii infection.Int Immunol. 2024 Apr 3;36(5):199-210. doi: 10.1093/intimm/dxae001. Int Immunol. 2024. PMID: 38175650 Free PMC article. Review.
-
Involvement of chemokine receptor CXCR3 in the defense mechanism against Neospora caninum infection in C57BL/6 mice.Front Microbiol. 2023 Jan 10;13:1045106. doi: 10.3389/fmicb.2022.1045106. eCollection 2022. Front Microbiol. 2023. PMID: 36704563 Free PMC article.
References
-
- Jones J, Lopez A, Wilson M. Congenital toxoplasmosis. Am Fam Physician. 2003;67:2131–2138. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

