Fibronectin precoating wound bed enhances the therapeutic effects of autologous epidermal basal cell suspension for full-thickness wounds by improving epidermal stem cells' utilization
- PMID: 31506090
- PMCID: PMC6737622
- DOI: 10.1186/s13287-019-1236-7
Fibronectin precoating wound bed enhances the therapeutic effects of autologous epidermal basal cell suspension for full-thickness wounds by improving epidermal stem cells' utilization
Abstract
Background: Autologous epidermal basal cell suspension therapy has been proven to be one of the most effective treatments for full-thickness wounds. However, we found there remain obvious defects that significantly confined the utilization and function of the epidermal basal cells (EBCs), especially the epidermal stem cells (ESCs) in it. This study investigated whether precoating fibronectin (FN) on the wound bed before spraying EBCs could overcome these defects and further explored its possible mechanisms.
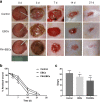
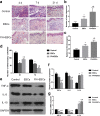
Methods: In the in vitro study, EBCs were isolated from the donor skin of patients who needed skin grafting. Different concentrations of FN were used to precoat culture dishes before cell culture; the adherent efficiency, proliferation and migration ability of ESCs were analyzed and compared with traditional collagen IV precoating. In the in vivo study, Sprague-Dawley (SD) rats with full-thickness skin wounds were selected as full-thickness wounds' model. For the experiment groups, 20 μg/ml FN was precoated on the wound bed 10 min before EBC spray. The quality of wound healing was estimated by the residual wound area rate, wound healing time, and hematoxylin and eosin (H&E) staining. Expression of ESC markers, neovascular markers, inflammation markers, and collagen formation and degradation markers was elucidated by immunohistochemistry (IHC), immunofluorescence (IF), western blot (WB), and RT-qPCR analysis.
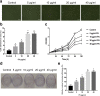
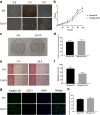
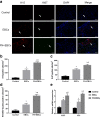
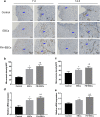
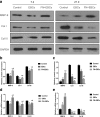
Results: The in vitro study showed that the dishes precoated with 20 μg/ml FN had a similar adherent efficiency and colony formation rate with collagen IV, but it could improve the proliferation and migration of ESCs significantly. Similarly, in the in vivo study, precoating FN on wound bed before EBC spray also significantly promote wound healing by improving ESCs' utilization efficiency, promoting angiogenesis, decreasing inflammations, and regulating collagen formation and degradation.
Conclusion: FN precoating wound bed before EBC spray could significantly promote full-thickness wound healing by improving the utilization and function of the ESCs and further by promoting angiogenesis, decreasing inflammations, and regulating collagen formation and degradation.
Keywords: Autologous EBC suspension therapy; Collagen IV; ESCs; FN; Full-thickness wounds.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Rat epidermal stem cells promote the angiogenesis of full-thickness wounds.Stem Cell Res Ther. 2020 Aug 8;11(1):344. doi: 10.1186/s13287-020-01844-y. Stem Cell Res Ther. 2020. PMID: 32771044 Free PMC article.
-
[Effects and mechanism of rat epidermal stem cells treated with exogenous vascular endothelial growth factor on healing of deep partial-thickness burn wounds in rats].Zhonghua Shao Shang Za Zhi. 2020 Mar 20;36(3):195-203. doi: 10.3760/cma.j.cn501120-20191125-00441. Zhonghua Shao Shang Za Zhi. 2020. PMID: 32241045 Chinese.
-
[Effects and mechanisms of polycaprolactone-cellulose acetate nanofiber scaffold loaded with rat epidermal stem cells on wound healing of full-thickness skin defects in rats].Zhonghua Shao Shang Za Zhi. 2021 May 20;37(5):460-468. doi: 10.3760/cma.j.cn501120-20210104-00005. Zhonghua Shao Shang Za Zhi. 2021. PMID: 33894697 Free PMC article. Chinese.
-
Activation of keratinocyte fibronectin receptor function during cutaneous wound healing.J Cell Sci Suppl. 1987;8:199-209. doi: 10.1242/jcs.1987.supplement_8.11. J Cell Sci Suppl. 1987. PMID: 2460476 Review.
-
[The modern approach to wound treatment].Med Pregl. 2000 Jul-Aug;53(7-8):363-8. Med Pregl. 2000. PMID: 11214479 Review. Croatian.
Cited by
-
Rat epidermal stem cells promote the angiogenesis of full-thickness wounds.Stem Cell Res Ther. 2020 Aug 8;11(1):344. doi: 10.1186/s13287-020-01844-y. Stem Cell Res Ther. 2020. PMID: 32771044 Free PMC article.
-
Long non‑coding RNA HOTAIR promotes burn wound healing by regulating epidermal stem cells.Mol Med Rep. 2020 Sep;22(3):1811-1820. doi: 10.3892/mmr.2020.11268. Epub 2020 Jun 23. Mol Med Rep. 2020. PMID: 32582996 Free PMC article.
-
[Research advances on skin tissue regeneration in wound repair].Zhonghua Shao Shang Za Zhi. 2021 Jul 20;37(7):670-674. doi: 10.3760/cma.j.cn501120-20200604-00296. Zhonghua Shao Shang Za Zhi. 2021. PMID: 34304409 Free PMC article. Review. Chinese.
-
Self-assembled biomimetic Nano-Matrix for stem cell anchorage.J Biomed Mater Res A. 2020 Apr;108(4):984-991. doi: 10.1002/jbm.a.36875. Epub 2020 Jan 10. J Biomed Mater Res A. 2020. PMID: 31904174 Free PMC article.
-
Galectin-3-integrin α5β1 phase separation disrupted by advanced glycation end-products impairs diabetic wound healing in rodents.Nat Commun. 2025 Aug 7;16(1):7287. doi: 10.1038/s41467-025-62320-w. Nat Commun. 2025. PMID: 40775187 Free PMC article.
References
-
- Wernick B, Stawicki SP. Impaired wound healing. Treasure Island: StatPearls; 2018. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

