Regulation of Skn7-dependent, oxidative stress-induced genes by the RNA polymerase II-CTD phosphatase, Fcp1, and Mediator kinase subunit, Cdk8, in yeast
- PMID: 31506296
- PMCID: PMC6827322
- DOI: 10.1074/jbc.RA119.008515
Regulation of Skn7-dependent, oxidative stress-induced genes by the RNA polymerase II-CTD phosphatase, Fcp1, and Mediator kinase subunit, Cdk8, in yeast
Abstract
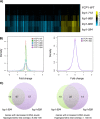
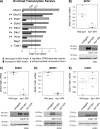
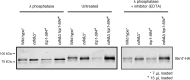
Fcp1 is a protein phosphatase that facilitates transcription elongation and termination by dephosphorylating the C-terminal domain of RNA polymerase II. High-throughput genetic screening and gene expression profiling of fcp1 mutants revealed a novel connection to Cdk8, the Mediator complex kinase subunit, and Skn7, a key transcription factor in the oxidative stress response pathway. Briefly, Skn7 was enriched as a regulator of genes whose mRNA levels were altered in fcp1 and cdk8Δ mutants and was required for the suppression of fcp1 mutant growth defects by loss of CDK8 under oxidative stress conditions. Targeted analysis revealed that mutating FCP1 decreased Skn7 mRNA and protein levels as well as its association with target gene promoters but paradoxically increased the mRNA levels of Skn7-dependent oxidative stress-induced genes (TRX2 and TSA1) under basal and induced conditions. The latter was in part recapitulated via chemical inhibition of transcription in WT cells, suggesting that a combination of transcriptional and posttranscriptional effects underscored the increased mRNA levels of TRX2 and TSA1 observed in the fcp1 mutant. Interestingly, loss of CDK8 robustly normalized the mRNA levels of Skn7-dependent genes in the fcp1 mutant background and also increased Skn7 protein levels by preventing its turnover. As such, our work suggested that loss of CDK8 could overcome transcriptional and/or posttranscriptional alterations in the fcp1 mutant through its regulatory effect on Skn7. Furthermore, our work also implicated FCP1 and CDK8 in the broader response to environmental stressors in yeast.
Keywords: Cdk8; Fcp1; Mediator complex; RNA polymerase II; Skn7; gene regulation; oxidative stress; transcription; transcription factor; transcription regulation; transcription repression; transcriptomics; yeast transcription.
© 2019 Aristizabal et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures





Similar articles
-
TORC1 signaling modulates Cdk8-dependent GAL gene expression in Saccharomyces cerevisiae.Genetics. 2021 Dec 10;219(4):iyab168. doi: 10.1093/genetics/iyab168. Genetics. 2021. PMID: 34849833 Free PMC article.
-
The Skn7 response regulator controls gene expression in the oxidative stress response of the budding yeast Saccharomyces cerevisiae.EMBO J. 1997 Mar 3;16(5):1035-44. doi: 10.1093/emboj/16.5.1035. EMBO J. 1997. PMID: 9118942 Free PMC article.
-
High-throughput genetic and gene expression analysis of the RNAPII-CTD reveals unexpected connections to SRB10/CDK8.PLoS Genet. 2013 Aug;9(8):e1003758. doi: 10.1371/journal.pgen.1003758. Epub 2013 Aug 29. PLoS Genet. 2013. PMID: 24009531 Free PMC article.
-
The two faces of Cdk8, a positive/negative regulator of transcription.Biochimie. 2014 Feb;97:22-7. doi: 10.1016/j.biochi.2013.10.004. Epub 2013 Oct 15. Biochimie. 2014. PMID: 24139904 Review.
-
The Mediator complex and transcription regulation.Crit Rev Biochem Mol Biol. 2013 Nov-Dec;48(6):575-608. doi: 10.3109/10409238.2013.840259. Epub 2013 Oct 3. Crit Rev Biochem Mol Biol. 2013. PMID: 24088064 Free PMC article. Review.
Cited by
-
H2O2 Induces Major Phosphorylation Changes in Critical Regulators of Signal Transduction, Gene Expression, Metabolism and Developmental Networks in Aspergillus nidulans.J Fungi (Basel). 2021 Jul 31;7(8):624. doi: 10.3390/jof7080624. J Fungi (Basel). 2021. PMID: 34436163 Free PMC article.
-
Oxidative stress response pathways in fungi.Cell Mol Life Sci. 2022 Jun 1;79(6):333. doi: 10.1007/s00018-022-04353-8. Cell Mol Life Sci. 2022. PMID: 35648225 Free PMC article. Review.
-
TORC1 signaling modulates Cdk8-dependent GAL gene expression in Saccharomyces cerevisiae.Genetics. 2021 Dec 10;219(4):iyab168. doi: 10.1093/genetics/iyab168. Genetics. 2021. PMID: 34849833 Free PMC article.
-
Mechanisms and Strategies for Engineering Oxidative Stress Resistance in Saccharomyces cerevisiae.Chem Bio Eng. 2025 May 29;2(7):409-422. doi: 10.1021/cbe.5c00021. eCollection 2025 Jul 24. Chem Bio Eng. 2025. PMID: 40735014 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

