Molecular identification of HSPA8 as an accessory protein of a hyperpolarization-activated chloride channel from rat pulmonary vein cardiomyocytes
- PMID: 31506297
- PMCID: PMC6827309
- DOI: 10.1074/jbc.RA119.007416
Molecular identification of HSPA8 as an accessory protein of a hyperpolarization-activated chloride channel from rat pulmonary vein cardiomyocytes
Abstract
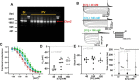
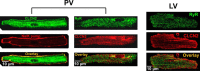
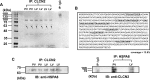
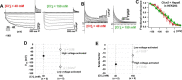
Pulmonary veins (PVs) are the major origin of atrial fibrillation. Recently, we recorded hyperpolarization-activated Cl- current (ICl, h) in rat PV cardiomyocytes. Unlike the well-known chloride channel protein 2 (CLCN2) current, the activation curve of ICl, h was hyperpolarized as the Cl- ion concentration ([Cl-] i ) increased. This current could account for spontaneous activity in PV cardiomyocytes linked to atrial fibrillation. In this study, we aimed to identify the channel underlying ICl, h Using RT-PCR amplification specific for Clcn2 or its homologs, a chloride channel was cloned from rat PV and detected in rat PV cardiomyocytes using immunocytochemistry. The gene sequence and electrophysiological functions of the protein were identical to those previously reported for Clcn2, with protein activity observed as a hyperpolarization-activated current by the patch-clamp method. However, the [Cl-] i dependence of activation was entirely different from the observed ICl, h of PV cardiomyocytes; the activation curve of the Clcn2-transfected cells shifted toward positive potential with increased [Cl-] i , whereas the ICl, h of PV and left ventricular cardiomyocytes showed a leftward shift. Therefore, we used MS to explore the possibility of additional proteins interacting with CLCN2 and identified an individual 71-kDa protein, HSPA8, that was strongly expressed in rat PV cardiomyocytes. With co-expression of HSPA8 in HEK293 and PC12 cells, the CLCN2 current showed voltage-dependent activation and shifted to negative potential with increasing [Cl-] i Molecular docking simulations further support an interaction between CLCN2 and HSPA8. These findings suggest that CLCN2 in rat heart contains HSPA8 as a unique accessory protein.
Keywords: cardiomyocyte; cardiovascular disease; chloride channel; heart; heat-shock protein (HSP); mass spectrometry (MS).
© 2019 Okamoto et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures






Similar articles
-
Pathological impact of hyperpolarization-activated chloride current peculiar to rat pulmonary vein cardiomyocytes.J Mol Cell Cardiol. 2014 Jan;66:53-62. doi: 10.1016/j.yjmcc.2013.11.002. Epub 2013 Nov 12. J Mol Cell Cardiol. 2014. PMID: 24239603
-
Caveolae-Mediated Activation of Mechanosensitive Chloride Channels in Pulmonary Veins Triggers Atrial Arrhythmogenesis.J Am Heart Assoc. 2019 Oct 15;8(20):e012748. doi: 10.1161/JAHA.119.012748. Epub 2019 Oct 10. J Am Heart Assoc. 2019. PMID: 31597508 Free PMC article.
-
Swelling activated chloride currents in the electrical activity of pulmonary vein cardiomyocytes.Eur J Clin Invest. 2008 Jan;38(1):17-23. doi: 10.1111/j.1365-2362.2007.01898.x. Eur J Clin Invest. 2008. PMID: 18173547
-
Comparative study of hyperpolarization-activated currents in pulmonary vein cardiomyocytes isolated from rat, guinea pig, and rabbit.J Physiol Sci. 2020 Feb 11;70(1):6. doi: 10.1186/s12576-020-00736-3. J Physiol Sci. 2020. PMID: 32046630 Free PMC article.
-
Ion currents, action potentials, and noradrenergic responses in rat pulmonary vein and left atrial cardiomyocytes.Physiol Rep. 2020 May;8(9):e14432. doi: 10.14814/phy2.14432. Physiol Rep. 2020. PMID: 32401431 Free PMC article.
Cited by
-
The Binding of HSPA8 and Mitochondrial ALDH2 Mediates Oxygen-Glucose Deprivation-Induced Fibroblast Senescence.Antioxidants (Basel). 2023 Dec 25;13(1):42. doi: 10.3390/antiox13010042. Antioxidants (Basel). 2023. PMID: 38247467 Free PMC article.
-
Association of heat shock protein polymorphisms with patient susceptibility to coronary artery disease comorbid depression and anxiety in a Chinese population.PeerJ. 2021 Jun 18;9:e11636. doi: 10.7717/peerj.11636. eCollection 2021. PeerJ. 2021. PMID: 34178482 Free PMC article.
-
Automaticity of the Pulmonary Vein Myocardium and the Effect of Class I Antiarrhythmic Drugs.Int J Mol Sci. 2024 Nov 18;25(22):12367. doi: 10.3390/ijms252212367. Int J Mol Sci. 2024. PMID: 39596432 Free PMC article. Review.
-
Effectiveness in the Block by Honokiol, a Dimerized Allylphenol from Magnolia Officinalis, of Hyperpolarization-Activated Cation Current and Delayed-Rectifier K+ Current.Int J Mol Sci. 2020 Jun 15;21(12):4260. doi: 10.3390/ijms21124260. Int J Mol Sci. 2020. PMID: 32549398 Free PMC article.
-
CryoEM structures of the human CLC-2 voltage-gated chloride channel reveal a ball-and-chain gating mechanism.Elife. 2024 Feb 12;12:RP90648. doi: 10.7554/eLife.90648. Elife. 2024. PMID: 38345841 Free PMC article.
References
-
- Haïssaguerre M., Jaïs P., Shah D. C., Takahashi A., Hocini M., Quiniou G., Garrigue S., Le Mouroux A., Le Métayer P., and Clémenty J. (1998) Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 339, 659–666 10.1056/NEJM199809033391003 - DOI - PubMed
-
- Chugh S. S., Havmoeller R., Narayanan K., Singh D., Rienstra M., Benjamin E. J., Gillum R. F., Kim Y. H., McAnulty J. H. Jr., Zheng Z. J., Forouzanfar M. H., Naghavi M., Mensah G. A., Ezzati M., and Murray C. J. (2014) Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation 129, 837–847 10.1161/CIRCULATIONAHA.113.005119 - DOI - PMC - PubMed
-
- Doisne N., Maupoil V., Cosnay P., and Findlay I. (2009) Catecholaminergic automatic activity in the rat pulmonary vein: electrophysiological differences between cardiac muscle in the left atrium and pulmonary vein. Am. J. Physiol. Heart Circ. Physiol. 297, H102–H108 10.1152/ajpheart.00256.2009 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

