Quorum Sensing and Metabolic State of the Host Control Lysogeny-Lysis Switch of Bacteriophage T1
- PMID: 31506310
- PMCID: PMC6737242
- DOI: 10.1128/mBio.01884-19
Quorum Sensing and Metabolic State of the Host Control Lysogeny-Lysis Switch of Bacteriophage T1
Abstract
Bacterial viruses, or bacteriophages, are highly abundant in the biosphere and have a major impact on microbial populations. Many examples of phage interactions with their hosts, including establishment of dormant lysogenic and active lytic states, have been characterized at the level of the individual cell. However, much less is known about the dependence of these interactions on host metabolism and signal exchange within bacterial communities. In this report, we describe a lysogenic state of the enterobacterial phage T1, previously known as a classical lytic phage, and characterize the underlying regulatory circuitry. We show that the transition from lysogeny to lysis depends on bacterial population density, perceived via interspecies autoinducer 2. Lysis is further controlled by the metabolic state of the cell, mediated by the cyclic-3',5'-AMP (cAMP) receptor protein (CRP) of the host. We hypothesize that such combinations of cell density and metabolic sensing may be common in phage-host interactions.IMPORTANCE The dynamics of microbial communities are heavily shaped by bacterium-bacteriophage interactions. But despite the apparent importance of bacteriophages, our understanding of the mechanisms controlling phage dynamics in bacterial populations, and particularly of the differences between the decisions that are made in the dormant lysogenic and active lytic states, remains limited. In this report, we show that enterobacterial phage T1, previously described as a lytic phage, is able to undergo lysogeny. We further demonstrate that the lysogeny-to-lysis decision occurs in response to changes in the density of the bacterial population, mediated by interspecies quorum-sensing signal AI-2, and in the metabolic state of the cell, mediated by cAMP receptor protein. We hypothesize that this strategy enables the phage to maximize its chances of self-amplification and spreading in bacterial population upon induction of the lytic cycle and that it might be common in phage-host interactions.
Keywords: Escherichia coli; bacteriophage lysis; cyclic AMP; quorum sensing.
Copyright © 2019 Laganenka et al.
Figures
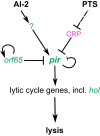
Comment in
-
Lysogeny of Escherichia coli by the Obligately Lytic Bacteriophage T1: Not Proven.mBio. 2021 May 4;12(3):e00740-21. doi: 10.1128/mBio.00740-21. mBio. 2021. PMID: 33947762 Free PMC article. No abstract available.
-
Reply to Jobling, "Lysogeny of Escherichia coli by the Obligately Lytic Bacteriophage T1: Not Proven".mBio. 2021 May 4;12(3):e01007-21. doi: 10.1128/mBio.01007-21. mBio. 2021. PMID: 33947764 Free PMC article. No abstract available.
Similar articles
-
The Role of Quorum Sensing in Phage Lifecycle Decision: A Switch Between Lytic and Lysogenic Pathways.Viruses. 2025 Feb 26;17(3):317. doi: 10.3390/v17030317. Viruses. 2025. PMID: 40143247 Free PMC article. Review.
-
The LuxO-OpaR quorum-sensing cascade differentially controls Vibriophage VP882 lysis-lysogeny decision making in liquid and on surfaces.PLoS Genet. 2024 Jul 30;20(7):e1011243. doi: 10.1371/journal.pgen.1011243. eCollection 2024 Jul. PLoS Genet. 2024. PMID: 39078816 Free PMC article.
-
A Host-Produced Quorum-Sensing Autoinducer Controls a Phage Lysis-Lysogeny Decision.Cell. 2019 Jan 10;176(1-2):268-280.e13. doi: 10.1016/j.cell.2018.10.059. Epub 2018 Dec 13. Cell. 2019. PMID: 30554875 Free PMC article.
-
Repeated outbreaks drive the evolution of bacteriophage communication.Elife. 2021 Jan 18;10:e58410. doi: 10.7554/eLife.58410. Elife. 2021. PMID: 33459590 Free PMC article.
-
Mechanisms of interactions between bacteria and bacteriophage mediate by quorum sensing systems.Appl Microbiol Biotechnol. 2022 Apr;106(7):2299-2310. doi: 10.1007/s00253-022-11866-6. Epub 2022 Mar 21. Appl Microbiol Biotechnol. 2022. PMID: 35312824 Review.
Cited by
-
Repurposing CRISPR-Cas Systems as Genetic Tools for the Enterobacteriales.EcoSal Plus. 2021 Dec 15;9(2):eESP00062020. doi: 10.1128/ecosalplus.ESP-0006-2020. Epub 2021 Jun 14. EcoSal Plus. 2021. PMID: 34125584 Free PMC article. Review.
-
Prophage Activation in the Intestine: Insights Into Functions and Possible Applications.Front Microbiol. 2021 Dec 13;12:785634. doi: 10.3389/fmicb.2021.785634. eCollection 2021. Front Microbiol. 2021. PMID: 34966370 Free PMC article. Review.
-
The Role of Quorum Sensing in Phage Lifecycle Decision: A Switch Between Lytic and Lysogenic Pathways.Viruses. 2025 Feb 26;17(3):317. doi: 10.3390/v17030317. Viruses. 2025. PMID: 40143247 Free PMC article. Review.
-
Phage therapy to treat cystic fibrosis Burkholderia cepacia complex lung infections: perspectives and challenges.Front Microbiol. 2024 Oct 18;15:1476041. doi: 10.3389/fmicb.2024.1476041. eCollection 2024. Front Microbiol. 2024. PMID: 39493847 Free PMC article. Review.
-
Separating Functions of the Phage-Encoded Quorum-Sensing-Activated Antirepressor Qtip.Cell Host Microbe. 2020 Apr 8;27(4):629-641.e4. doi: 10.1016/j.chom.2020.01.024. Epub 2020 Feb 25. Cell Host Microbe. 2020. PMID: 32101705 Free PMC article.
References
-
- Zhan L, Han Y, Yang L, Geng J, Li Y, Gao H, Guo Z, Fan W, Li G, Zhang L, Qin C, Zhou D, Yang R. 2008. The cyclic AMP receptor protein, CRP, is required for both virulence and expression of the minimal CRP regulon in Yersinia pestis biovar microtus. Infect Immun 76:5028–5037. doi:10.1128/IAI.00370-08. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

