Emergent Properties in Streptococcus mutans Biofilms Are Controlled through Adhesion Force Sensing by Initial Colonizers
- PMID: 31506311
- PMCID: PMC6737243
- DOI: 10.1128/mBio.01908-19
Emergent Properties in Streptococcus mutans Biofilms Are Controlled through Adhesion Force Sensing by Initial Colonizers
Abstract
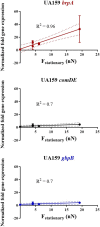
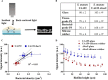
Bacterial adhesion is accompanied by altered gene expression, leading to "emergent" properties of biofilm bacteria that are alien to planktonic ones. With the aim of revealing the role of environmental adhesion forces in emergent biofilm properties, genes in Streptococcus mutans UA159 and a quorum-sensing-deficient mutant were identified that become expressed after adhesion to substratum surfaces. Using atomic force microscopy, adhesion forces of initial S. mutans colonizers on four different substrata were determined and related to gene expression. Adhesion forces upon initial contact were similarly low across different substrata, ranging between 0.2 and 1.2 nN regardless of the strain considered. Bond maturation required up to 21 s, depending on the strain and substratum surface involved, but stationary adhesion forces also were similar in the parent and in the mutant strain. However, stationary adhesion forces were largest on hydrophobic silicone rubber (19 to 20 nN), while being smallest on hydrophilic glass (3 to 4 nN). brpA gene expression in thin (34 to 48 μm) 5-h S. mutans UA159 biofilms was most sensitive to adhesion forces, while expression of gbpB and comDE expressions was weakly sensitive. ftf, gtfB, vicR, and relA expression was insensitive to adhesion forces. In thicker (98 to 151 μm) 24-h biofilms, adhesion-force-induced gene expression and emergent extracellular polymeric substance (EPS) production were limited to the first 20 to 30 μm above a substratum surface. In the quorum-sensing-deficient S. mutans, adhesion-force-controlled gene expression was absent in both 5- and 24-h biofilms. Thus, initial colonizers of substratum surfaces sense adhesion forces that externally trigger emergent biofilm properties over a limited distance above a substratum surface through quorum sensing.IMPORTANCE A new concept in biofilm science is introduced: "adhesion force sensitivity of genes," defining the degree up to which expression of different genes in adhering bacteria is controlled by the environmental adhesion forces they experience. Analysis of gene expression as a function of height in a biofilm showed that the information about the substratum surface to which initially adhering bacteria adhere is passed up to a biofilm height of 20 to 30 μm above a substratum surface, highlighting the importance and limitations of cell-to-cell communication in a biofilm. Bacteria in a biofilm mode of growth, as opposed to planktonic growth, are responsible for the great majority of human infections, predicted to become the number one cause of death in 2050. The concept of adhesion force sensitivity of genes provides better understanding of bacterial adaptation in biofilms, direly needed for the design of improved therapeutic measures that evade the recalcitrance of biofilm bacteria to antimicrobials.
Keywords: OCT; atomic force microscopy; quorum sensing; regulation of gene expression; surface sensing.
Copyright © 2019 Wang et al.
Figures

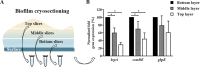
References
-
- Hermansson M. 1999. The DLVO theory in microbial adhesion. Colloids Surf B Biointerfaces 14:105–119. doi:10.1016/S0927-7765(99)00029-6. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
