The single-cell transcriptomic landscape of early human diabetic nephropathy
- PMID: 31506348
- PMCID: PMC6765272
- DOI: 10.1073/pnas.1908706116
The single-cell transcriptomic landscape of early human diabetic nephropathy
Abstract
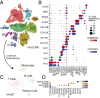
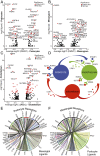
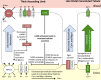
Diabetic nephropathy is characterized by damage to both the glomerulus and tubulointerstitium, but relatively little is known about accompanying cell-specific changes in gene expression. We performed unbiased single-nucleus RNA sequencing (snRNA-seq) on cryopreserved human diabetic kidney samples to generate 23,980 single-nucleus transcriptomes from 3 control and 3 early diabetic nephropathy samples. All major cell types of the kidney were represented in the final dataset. Side-by-side comparison demonstrated cell-type-specific changes in gene expression that are important for ion transport, angiogenesis, and immune cell activation. In particular, we show that the diabetic thick ascending limb, late distal convoluted tubule, and principal cells all adopt a gene expression signature consistent with increased potassium secretion, including alterations in Na+/K+-ATPase, WNK1, mineralocorticoid receptor, and NEDD4L expression, as well as decreased paracellular calcium and magnesium reabsorption. We also identify strong angiogenic signatures in glomerular cell types, proximal convoluted tubule, distal convoluted tubule, and principal cells. Taken together, these results suggest that increased potassium secretion and angiogenic signaling represent early kidney responses in human diabetic nephropathy.
Keywords: RNA-seq; diabetic nephropathy; single cell.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
Conflict of interest statement: In separate work, B.D.H. consults for and receives grant support from Janssen to study mouse diabetic nephropathy. B.D.H. also consults for and holds equity in Chinook Therapeutics. Neither relationship is related to the contents of this manuscript.
Figures


References
-
- Wilson P. C., Humphreys B. D., Single-cell genomics and gene editing: Implications for nephrology. Nat. Rev. Nephrol. 15, 63–64 (2019). - PubMed
-
- Baelde H. J., et al. , Gene expression profiling in glomeruli from human kidneys with diabetic nephropathy. Am. J. Kidney Dis. 43, 636–650 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

