Circular code motifs in the ribosome: a missing link in the evolution of translation?
- PMID: 31506380
- PMCID: PMC6859856
- DOI: 10.1261/rna.072074.119
Circular code motifs in the ribosome: a missing link in the evolution of translation?
Abstract
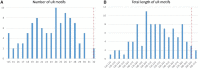
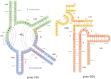
The origin of the genetic code remains enigmatic five decades after it was elucidated, although there is growing evidence that the code coevolved progressively with the ribosome. A number of primordial codes were proposed as ancestors of the modern genetic code, including comma-free codes such as the RRY, RNY, or GNC codes (R = G or A, Y = C or T, N = any nucleotide), and the X circular code, an error-correcting code that also allows identification and maintenance of the reading frame. It was demonstrated previously that motifs of the X circular code are significantly enriched in the protein-coding genes of most organisms, from bacteria to eukaryotes. Here, we show that imprints of this code also exist in the ribosomal RNA (rRNA). In a large-scale study involving 133 organisms representative of the three domains of life, we identified 32 universal X motifs that are conserved in the rRNA of >90% of the organisms. Intriguingly, most of the universal X motifs are located in rRNA regions involved in important ribosome functions, notably in the peptidyl transferase center and the decoding center that form the original "proto-ribosome." Building on the existing accretion models for ribosome evolution, we propose that error-correcting circular codes represented an important step in the emergence of the modern genetic code. Thus, circular codes would have allowed the simultaneous coding of amino acids and synchronization of the reading frame in primitive translation systems, prior to the emergence of more sophisticated start codon recognition and translation initiation mechanisms.
Keywords: circular code; genetic code; origin of life; ribosome evolution; translation.
© 2019 Dila et al.; Published by Cold Spring Harbor Laboratory Press for the RNA Society.
Figures


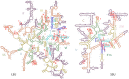


Similar articles
-
Identification of a circular code periodicity in the bacterial ribosome: origin of codon periodicity in genes?RNA Biol. 2020 Apr;17(4):571-583. doi: 10.1080/15476286.2020.1719311. Epub 2020 Feb 11. RNA Biol. 2020. PMID: 31960748 Free PMC article.
-
Potential role of the X circular code in the regulation of gene expression.Biosystems. 2021 May;203:104368. doi: 10.1016/j.biosystems.2021.104368. Epub 2021 Feb 7. Biosystems. 2021. PMID: 33567309
-
Circular code motifs in the ribosome decoding center.Comput Biol Chem. 2014 Oct;52:9-17. doi: 10.1016/j.compbiolchem.2014.08.001. Epub 2014 Aug 5. Comput Biol Chem. 2014. PMID: 25215650
-
The ribosome: a molecular machine powered by RNA.Met Ions Life Sci. 2011;9:253-75. doi: 10.1039/9781849732512-00253. Met Ions Life Sci. 2011. PMID: 22010275 Review.
-
Structural dynamics of ribosomal RNA during decoding on the ribosome.Biochimie. 2002 Aug;84(8):745-54. doi: 10.1016/s0300-9084(02)01409-8. Biochimie. 2002. PMID: 12457562 Review.
Cited by
-
Circular RNAs: A New Piece in the Colorectal Cancer Puzzle.Cancers (Basel). 2020 Aug 31;12(9):2464. doi: 10.3390/cancers12092464. Cancers (Basel). 2020. PMID: 32878117 Free PMC article. Review.
-
The primordial tRNA acceptor stem code from theoretical minimal RNA ring clusters.BMC Genet. 2020 Jan 23;21(1):7. doi: 10.1186/s12863-020-0812-2. BMC Genet. 2020. PMID: 31973715 Free PMC article.
-
Combinatorial Fusion Rules to Describe Codon Assignment in the Standard Genetic Code.Life (Basel). 2020 Dec 23;11(1):4. doi: 10.3390/life11010004. Life (Basel). 2020. PMID: 33374866 Free PMC article.
-
Footprints of a Singular 22-Nucleotide RNA Ring at the Origin of Life.Biology (Basel). 2020 Apr 25;9(5):88. doi: 10.3390/biology9050088. Biology (Basel). 2020. PMID: 32344921 Free PMC article.
-
Origin of Life: The Point of No Return.Life (Basel). 2020 Nov 3;10(11):269. doi: 10.3390/life10110269. Life (Basel). 2020. PMID: 33153087 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
