Omega-3 fatty acids decrease oxidative stress and inflammation in macrophages from patients with small abdominal aortic aneurysm
- PMID: 31506475
- PMCID: PMC6736886
- DOI: 10.1038/s41598-019-49362-z
Omega-3 fatty acids decrease oxidative stress and inflammation in macrophages from patients with small abdominal aortic aneurysm
Abstract
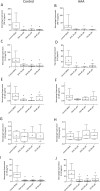
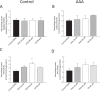
Abdominal aortic aneurysm (AAA) is associated with inflammation and oxidative stress, the latter of which contributes to activation of macrophages, a prominent cell type in AAA. Omega-3 polyunsaturated fatty acids (n-3 PUFAs) have been reported to limit oxidative stress in animal models of AAA. The aim of this study was to evaluate the effect of the n-3 PUFA docosahexaenoic acid (DHA) on antioxidant defence in macrophages from patients with AAA. Cells were obtained from men with small AAA (diameter 3.0-4.5 cm, 75 ± 6 yr, n = 19) and age- matched male controls (72 ± 5 yr, n = 41) and incubated with DHA for 1 h before exposure to 0.1 µg/mL lipopolysaccharide (LPS) for 24 h. DHA supplementation decreased the concentration of tumour necrosis factor-α (TNF-α; control, 42.1 ± 13.6 to 5.1 ± 2.1 pg/ml, p < 0.01; AAA, 25.2 ± 9.8 to 1.9 ± 0.9 pg/ml, p < 0.01) and interleukin-6 (IL-6; control, 44.9 ± 7.7 to 5.9 ± 2.0 pg/ml, p < 0.001; AAA, 24.3 ± 5.2 to 0.5 ± 0.3 pg/ml, p < 0.001) in macrophage supernatants. DHA increased glutathione peroxidase activity (control, 3.2 ± 0.3 to 4.1 ± 0.2 nmol/min/ml/μg protein, p = 0.004; AAA, 2.3 ± 0.5 to 3.4 ± 0.5 nmol/min/ml/μg protein, p = 0.008) and heme oxygenase-1 mRNA expression (control, 1.5-fold increase, p < 0.001). The improvements in macrophage oxidative stress status serve as a stimulus for further investigation of DHA in patients with AAA.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
n-3 PUFAs improve erythrocyte fatty acid profile in patients with small AAA: a randomized controlled trial.J Lipid Res. 2019 Jun;60(6):1154-1163. doi: 10.1194/jlr.P093013. Epub 2019 Mar 26. J Lipid Res. 2019. PMID: 30914500 Free PMC article. Clinical Trial.
-
Omega 3 Polyunsaturated Fatty Acids Suppress the Development of Aortic Aneurysms Through the Inhibition of Macrophage-Mediated Inflammation.Circ J. 2015;79(7):1470-8. doi: 10.1253/circj.CJ-14-0471. Epub 2015 Apr 30. Circ J. 2015. PMID: 25925976
-
Resveratrol prevents the development of abdominal aortic aneurysm through attenuation of inflammation, oxidative stress, and neovascularization.Atherosclerosis. 2011 Aug;217(2):350-7. doi: 10.1016/j.atherosclerosis.2011.03.042. Epub 2011 Apr 8. Atherosclerosis. 2011. PMID: 21530968
-
Abdominal aortic aneurysm and omega-3 polyunsaturated fatty acids: Mechanisms, animal models, and potential treatment.Prostaglandins Leukot Essent Fatty Acids. 2017 Mar;118:1-9. doi: 10.1016/j.plefa.2017.02.001. Epub 2017 Feb 10. Prostaglandins Leukot Essent Fatty Acids. 2017. PMID: 28288701 Review.
-
n-3 Polyunsaturated fatty acids for the management of alcoholic liver disease: A critical review.Crit Rev Food Sci Nutr. 2019;59(sup1):S116-S129. doi: 10.1080/10408398.2018.1544542. Epub 2018 Dec 22. Crit Rev Food Sci Nutr. 2019. PMID: 30580553 Review.
Cited by
-
Serum Oxylipin Profiles Identify Potential Biomarkers in Patients with Acute Aortic Dissection.Metabolites. 2022 Jun 23;12(7):587. doi: 10.3390/metabo12070587. Metabolites. 2022. PMID: 35888709 Free PMC article.
-
Study protocol of a randomized controlled clinical trial investigating the effects of omega-3 supplementation on endothelial function, vascular structure, and metabolic parameters in adolescents with type 1 diabetes.Trials. 2021 Dec 27;22(1):953. doi: 10.1186/s13063-021-05930-1. Trials. 2021. PMID: 34961564 Free PMC article.
-
Therapeutic Potential of Heme Oxygenase-1 in Aneurysmal Diseases.Antioxidants (Basel). 2020 Nov 19;9(11):1150. doi: 10.3390/antiox9111150. Antioxidants (Basel). 2020. PMID: 33228202 Free PMC article. Review.
-
Anti-Inflammatory Effects of (9Z,11E)-13-Oxooctadeca-9,11-dienoic Acid (13-KODE) Derived from Salicornia herbacea L. on Lipopolysaccharide-Stimulated Murine Macrophage via NF-kB and MAPK Inhibition and Nrf2/HO-1 Signaling Activation.Antioxidants (Basel). 2022 Jan 18;11(2):180. doi: 10.3390/antiox11020180. Antioxidants (Basel). 2022. PMID: 35204063 Free PMC article.
-
Microalgae as a Nutraceutical Tool to Antagonize the Impairment of Redox Status Induced by SNPs: Implications on Insulin Resistance.Biology (Basel). 2023 Mar 15;12(3):449. doi: 10.3390/biology12030449. Biology (Basel). 2023. PMID: 36979141 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

