Dynamics of centriole amplification in centrosome-depleted brain multiciliated progenitors
- PMID: 31506528
- PMCID: PMC6736942
- DOI: 10.1038/s41598-019-49416-2
Dynamics of centriole amplification in centrosome-depleted brain multiciliated progenitors
Abstract
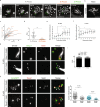
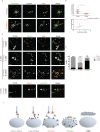
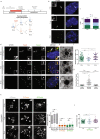
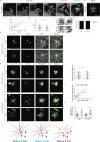
Reproductive and respiratory organs, along with brain ventricles, are lined by multiciliated epithelial cells (MCC) that generate cilia-powered fluid flows. MCC hijack the centrosome duplication pathway to form hundreds of centrioles and nucleate motile cilia. In these cells, the large majority of procentrioles are formed associated with partially characterized organelles called deuterosomes. We recently challenged the paradigm that deuterosomes and procentrioles are formed de novo by providing data, in brain MCC, suggesting that they are nucleated from the pre-existing centrosomal younger centriole. However, the origin of deuterosomes and procentrioles is still under debate. Here, we further question centrosome importance for deuterosome and centriole amplification. First, we provide additional data confirming that centriole amplification occurs sequentially from the centrosomal region, and that the first procentriole-loaded deuterosomes are associated with the daughter centriole or in the centrosomal centriole vicinity. Then, to further test the requirement of the centrosome in deuterosome and centriole formation, we depleted centrosomal centrioles using a Plk4 inhibitor. We reveal unexpected limited consequences in deuterosome/centriole number in absence of centrosomal centrioles. Notably, in absence of the daughter centriole only, deuterosomes are not seen associated with the mother centriole. In absence of both centrosomal centrioles, procentrioles are still amplified sequentially and with no apparent structural defects. They seem to arise from a focal region, characterized by microtubule convergence and pericentriolar material (PCM) assembly. The relevance of deuterosome association with the daughter centriole as well as the role of the PCM in the focal and sequential genesis of centrioles in absence of centrosomal centrioles are discussed.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Centriole amplification by mother and daughter centrioles differs in multiciliated cells.Nature. 2014 Dec 4;516(7529):104-7. doi: 10.1038/nature13770. Epub 2014 Oct 12. Nature. 2014. PMID: 25307055
-
Regulation of cilia abundance in multiciliated cells.Elife. 2019 Apr 26;8:e44039. doi: 10.7554/eLife.44039. Elife. 2019. PMID: 31025935 Free PMC article.
-
PLK4 drives centriole amplification and apical surface area expansion in multiciliated cells.Elife. 2022 Aug 15;11:e80643. doi: 10.7554/eLife.80643. Elife. 2022. PMID: 35969030 Free PMC article.
-
Building a centriole.Curr Opin Cell Biol. 2013 Feb;25(1):72-7. doi: 10.1016/j.ceb.2012.10.016. Epub 2012 Nov 27. Curr Opin Cell Biol. 2013. PMID: 23199753 Free PMC article. Review.
-
Emerging Picture of Deuterosome-Dependent Centriole Amplification in MCCs.Cells. 2018 Sep 27;7(10):152. doi: 10.3390/cells7100152. Cells. 2018. PMID: 30262752 Free PMC article. Review.
Cited by
-
Evolutionary conservation of centriole rotational asymmetry in the human centrosome.Elife. 2022 Mar 23;11:e72382. doi: 10.7554/eLife.72382. Elife. 2022. PMID: 35319462 Free PMC article.
-
Fibrogranular materials function as organizers to ensure the fidelity of multiciliary assembly.Nat Commun. 2021 Feb 24;12(1):1273. doi: 10.1038/s41467-021-21506-8. Nat Commun. 2021. PMID: 33627667 Free PMC article.
-
Formation and function of multiciliated cells.J Cell Biol. 2024 Jan 1;223(1):e202307150. doi: 10.1083/jcb.202307150. Epub 2023 Nov 30. J Cell Biol. 2024. PMID: 38032388 Free PMC article. Review.
-
Advances in Understanding the Genetic Mechanisms of Zebrafish Renal Multiciliated Cell Development.J Dev Biol. 2022 Dec 21;11(1):1. doi: 10.3390/jdb11010001. J Dev Biol. 2022. PMID: 36648903 Free PMC article. Review.
-
Deup1 Expression Interferes with Multiciliated Differentiation.Mol Cells. 2023 Dec 31;46(12):746-756. doi: 10.14348/molcells.2023.0149. Epub 2023 Nov 8. Mol Cells. 2023. PMID: 38052490 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

