The double inhibition of PDK1 and STAT3-Y705 prevents liver metastasis in colorectal cancer
- PMID: 31506552
- PMCID: PMC6736869
- DOI: 10.1038/s41598-019-49480-8
The double inhibition of PDK1 and STAT3-Y705 prevents liver metastasis in colorectal cancer
Abstract
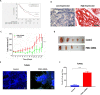
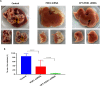
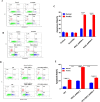
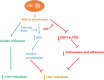
As a key glycolysis enzyme, the significance of pyruvate dehydrogenase kinase 1 (PDK1) in the development of colorectal cancer (CRC) remains unknown. This study revealed that the prognosis of CRC patients with high levels of PDK1 was poor, and PDK1 knockdown significantly reduced liver metastasis of CRC in both nude mice and immune competent BALB/C mice. When combined with cryptotanshinone (CPT), an inhibitor of STAT3-p-Y705, the liver metastasis was further inhibited. PDK1 knockdown obviously increased reactive oxygen species level in anoikis conditions and subsequently resulted in an elevated anoikis, but the combination of PDK1 knockdown and CPT showed a reduced effect on anoikis. Based on this discrepancy, the adherence ability of CRC cells to matrix protein fibronectin was further detected. It showed that PDK1 knockdown significantly decreased the adherence of CRC cells to fibronectin when combined with CPT. These results suggest that inhibition of PDK1 can decrease the surviving CRC cells in blood circulation via up-regulation of anoikis, and inhibition of STAT3-p-Y705 can prevent it to settle down on the liver premetastatic niche, which ultimately reduces liver metastasis.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Mutant KRAS promotes liver metastasis of colorectal cancer, in part, by upregulating the MEK-Sp1-DNMT1-miR-137-YB-1-IGF-IR signaling pathway.Oncogene. 2018 Jun;37(25):3440-3455. doi: 10.1038/s41388-018-0222-3. Epub 2018 Mar 21. Oncogene. 2018. PMID: 29559746
-
CPEB3 inhibits epithelial-mesenchymal transition by disrupting the crosstalk between colorectal cancer cells and tumor-associated macrophages via IL-6R/STAT3 signaling.J Exp Clin Cancer Res. 2020 Jul 11;39(1):132. doi: 10.1186/s13046-020-01637-4. J Exp Clin Cancer Res. 2020. PMID: 32653013 Free PMC article.
-
Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis.Mol Cancer. 2019 Mar 30;18(1):64. doi: 10.1186/s12943-019-0976-4. Mol Cancer. 2019. PMID: 30927925 Free PMC article.
-
Emerging role of bile acids in colorectal liver metastasis: From molecular mechanism to clinical significance (Review).Int J Oncol. 2025 Mar;66(3):24. doi: 10.3892/ijo.2025.5730. Epub 2025 Feb 21. Int J Oncol. 2025. PMID: 39981904 Free PMC article. Review.
-
Targeting key transcriptional factor STAT3 in colorectal cancer.Mol Cell Biochem. 2021 Sep;476(9):3219-3228. doi: 10.1007/s11010-021-04156-8. Epub 2021 Apr 18. Mol Cell Biochem. 2021. PMID: 33866491 Review.
Cited by
-
Ribophorin II promotes the epithelial-mesenchymal transition and aerobic glycolysis of laryngeal squamous cell carcinoma via regulating reactive oxygen species-mediated Phosphatidylinositol-3-Kinase/Protein Kinase B activation.Bioengineered. 2022 Mar;13(3):5141-5151. doi: 10.1080/21655979.2022.2036914. Bioengineered. 2022. PMID: 35156537 Free PMC article.
-
The Implications of PDK1-4 on Tumor Energy Metabolism, Aggressiveness and Therapy Resistance.Front Oncol. 2020 Dec 15;10:583217. doi: 10.3389/fonc.2020.583217. eCollection 2020. Front Oncol. 2020. PMID: 33384955 Free PMC article. Review.
-
Cancer Stem Cell-Associated Pathways in the Metabolic Reprogramming of Breast Cancer.Int J Mol Sci. 2020 Nov 30;21(23):9125. doi: 10.3390/ijms21239125. Int J Mol Sci. 2020. PMID: 33266219 Free PMC article. Review.
-
Non-metabolic enzyme function of pyruvate kinase M2 in breast cancer.Front Oncol. 2024 Oct 1;14:1450325. doi: 10.3389/fonc.2024.1450325. eCollection 2024. Front Oncol. 2024. PMID: 39411137 Free PMC article. Review.
-
Vitamin C activates pyruvate dehydrogenase (PDH) targeting the mitochondrial tricarboxylic acid (TCA) cycle in hypoxic KRAS mutant colon cancer.Theranostics. 2021 Jan 25;11(8):3595-3606. doi: 10.7150/thno.51265. eCollection 2021. Theranostics. 2021. PMID: 33664850 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

