Effects of Sodium-Glucose Cotransporter 2 Inhibitors on Renal Outcomes in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
- PMID: 31506585
- PMCID: PMC6736944
- DOI: 10.1038/s41598-019-49525-y
Effects of Sodium-Glucose Cotransporter 2 Inhibitors on Renal Outcomes in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
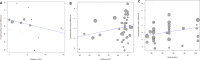
This study was conducted to investigate the effects of sodium-glucose cotransporter 2 (SGLT2) inhibitors on individual renal outcomes in patients with type 2 diabetes. We searched MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials from inception to September 2017 to identify randomized controlled trials comparing SGLT2 inhibitors with placebo or antidiabetic drugs and reporting any renal outcomes in patients with type 2 diabetes. Additionally, we identified 4 articles which were published after the predefined period to include relevant data. A meta-analysis was performed to calculate weighted mean differences (WMDs) and relative risks (RRs) with 95% confidence intervals (CIs) for each renal outcome. We included 48 studies involving 58,165 patients in the analysis. SGLT2 inhibitors significantly lowered urine albumin-to-creatinine ratio (UACR) (WMD, -14.64 mg/g; 95% CI, -25.15 to -4.12; P = 0.006) compared with controls. The UACR-lowering effects of SGLT2 inhibitors were greater with a higher baseline UACR. Overall changes in estimated glomerular filtration rate (eGFR) were comparable between two groups (WMD, 0.19 mL/min/1.73 m2; 95% CI, -0.44 to 0.82; P = 0.552). However, SGLT2 inhibitors significantly slowed eGFR decline in patients with a higher baseline eGFR and a longer duration of treatment. Compared with controls, SGLT2 inhibitors significantly reduced the risk of microalbuminuria (RR, 0.69; 95% CI, 0.49 to 0.97; P = 0.032), macroalbuminuria (RR, 0.49; 95% CI, 0.33 to 0.73; P < 0.001), and worsening nephropathy (RR, 0.73; 95% CI, 0.58 to 0.93; P = 0.012). In addition, the risk of end-stage renal disease was significantly lower in SGLT2 inhibitors than in controls (RR, 0.70; 95% CI, 0.57 to 0.87; P = 0.001). In conclusion, SGLT2 inhibitors had beneficial renal effects by lowering the risk of albuminuria development or progression and reducing the risk of end-stage renal disease compared with placebo or other antidiabetic drugs.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Effects of Dipeptidyl Peptidase-4 Inhibitors on Renal Outcomes in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis.Endocrinol Metab (Seoul). 2019 Mar;34(1):80-92. doi: 10.3803/EnM.2019.34.1.80. Endocrinol Metab (Seoul). 2019. PMID: 30912341 Free PMC article.
-
The renoprotective effects of sodium-glucose cotransporter 2 inhibitors versus placebo in patients with type 2 diabetes with or without prevalent kidney disease: A systematic review and meta-analysis.Diabetes Obes Metab. 2019 Apr;21(4):1018-1026. doi: 10.1111/dom.13620. Epub 2019 Jan 16. Diabetes Obes Metab. 2019. PMID: 30565382
-
SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis.Lancet Diabetes Endocrinol. 2019 Nov;7(11):845-854. doi: 10.1016/S2213-8587(19)30256-6. Epub 2019 Sep 5. Lancet Diabetes Endocrinol. 2019. PMID: 31495651
-
Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: A systematic review and meta-analysis.Diabetes Obes Metab. 2019 May;21(5):1237-1250. doi: 10.1111/dom.13648. Epub 2019 Mar 4. Diabetes Obes Metab. 2019. PMID: 30697905
-
Effect of SGLT2 inhibitor on renal function in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials.Int Urol Nephrol. 2019 Apr;51(4):655-669. doi: 10.1007/s11255-019-02112-6. Epub 2019 Mar 4. Int Urol Nephrol. 2019. PMID: 30830656
Cited by
-
Metabolic Impact of Frailty Changes Diabetes Trajectory.Metabolites. 2023 Feb 16;13(2):295. doi: 10.3390/metabo13020295. Metabolites. 2023. PMID: 36837914 Free PMC article. Review.
-
Sodium Glucose Cotransporter-2 Inhibitor Protects Against Diabetic Neuropathy and Nephropathy in Modestly Controlled Type 2 Diabetes: Follow-Up Study.Front Endocrinol (Lausanne). 2022 Jun 17;13:864332. doi: 10.3389/fendo.2022.864332. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35784562 Free PMC article.
-
Mechanisms and Perspectives of Sodium-Glucose Co-transporter 2 Inhibitors in Heart Failure.Front Cardiovasc Med. 2021 Feb 10;8:636152. doi: 10.3389/fcvm.2021.636152. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 33644138 Free PMC article. Review.
-
SGLT2 Inhibitors and Kidney Outcomes in Patients with Chronic Kidney Disease.J Clin Med. 2020 Aug 24;9(9):2723. doi: 10.3390/jcm9092723. J Clin Med. 2020. PMID: 32846935 Free PMC article.
-
Changes in the estimated glomerular filtration rate and predictors of the renal prognosis in Japanese patients with type 2 diabetes: A retrospective study during the 12 months after the initiation of tofogliflozin.PLoS One. 2023 Sep 21;18(9):e0292014. doi: 10.1371/journal.pone.0292014. eCollection 2023. PLoS One. 2023. PMID: 37733761 Free PMC article.
References
-
- Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus: Cardiovascular and Kidney Effects, Potential Mechanisms, and Clinical Applications. Circulation. 2016;134:752–772. doi: 10.1161/CIRCULATIONAHA.116.021887. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

