Design, synthesis, in silico and in vitro evaluation of novel diphenyl ether derivatives as potential antitubercular agents
- PMID: 31506871
- PMCID: PMC11177332
- DOI: 10.1007/s11030-019-09990-z
Design, synthesis, in silico and in vitro evaluation of novel diphenyl ether derivatives as potential antitubercular agents
Abstract
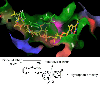
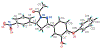
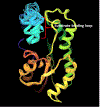
Diphenyl ether derivatives inhibit mycobacterial cell wall synthesis by inhibiting an enzyme, enoyl-acyl carrier protein reductase (InhA), which catalyses the last step in the fatty acid synthesis cycle of genus Mycobacterium. To select and validate a protein crystal structure of enoyl-acyl carrier protein reductase of Mycobacterium tuberculosis for designing inhibitors using molecular modelling, a cross-docking and correlation study was performed. A series of novel 1-(3-(3-hydroxy-4-phenoxyphenyl)-5-phenyl-4,5-dihydro-1H-pyrazol-1-yl) ethan-1-ones were synthesized from this model and screened for their antitubercular activity against M. tuberculosis H37Rv. Compound PYN-8 showed good antitubercular activity on M. tuberculosis H37Rv (MIC = 4-7 µM) and Mycobacterium bovis (% inhibition at 10 µM = 95.91%). Cytotoxicity of all the synthesized derivatives was assessed using various cell lines, and they were found to be safe. Structure of PYN-8 was also confirmed by single-crystal X-ray diffraction. The molecular modelling studies also corroborated the biological activity of the compounds. Further, in silico findings revealed that all these tested compounds exhibited good ADME properties and drug likeness and thus may be considered as potential candidates for further drug development.
Keywords: Antitubercular; Correlation study; Diphenylether; InhA; Molecular docking; TB.
Conflict of interest statement
Conflict of Interest
The authors confirm that this article content has no conflict of interest.
Figures



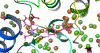

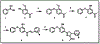
Similar articles
-
Rational design and synthesis of novel diphenyl ether derivatives as antitubercular agents.Drug Des Devel Ther. 2016 Jul 18;10:2299-310. doi: 10.2147/DDDT.S104037. eCollection 2016. Drug Des Devel Ther. 2016. PMID: 27486307 Free PMC article.
-
Synthesis, Biological Evaluation and Molecular Docking Studies of New Pyrazolines as an Antitubercular and Cytotoxic Agents.Infect Disord Drug Targets. 2019;19(3):310-321. doi: 10.2174/1871526519666181217120626. Infect Disord Drug Targets. 2019. PMID: 30556506
-
Design and synthesis of thiourea-based derivatives as Mycobacterium tuberculosis growth and enoyl acyl carrier protein reductase (InhA) inhibitors.Eur J Med Chem. 2020 Aug 1;199:112402. doi: 10.1016/j.ejmech.2020.112402. Epub 2020 May 4. Eur J Med Chem. 2020. PMID: 32417538
-
Recent Advances and Structural Features of Enoyl-ACP Reductase Inhibitors of Mycobacterium tuberculosis.Arch Pharm (Weinheim). 2016 Nov;349(11):817-826. doi: 10.1002/ardp.201600186. Epub 2016 Oct 24. Arch Pharm (Weinheim). 2016. PMID: 27775177 Review.
-
Mycobacterium enoyl acyl carrier protein reductase (InhA): A key target for antitubercular drug discovery.Bioorg Chem. 2021 Oct;115:105242. doi: 10.1016/j.bioorg.2021.105242. Epub 2021 Aug 8. Bioorg Chem. 2021. PMID: 34392175 Review.
Cited by
-
Molecular insights into Mmpl3 leads to the development of novel indole-2-carboxamides as antitubercular agents.Mol Syst Des Eng. 2022 Jun 1;7(6):592-606. doi: 10.1039/d1me00122a. Epub 2022 Mar 2. Mol Syst Des Eng. 2022. PMID: 36186547 Free PMC article.
-
Developing novel indoles as antitubercular agents and simulated annealing-based analysis of their binding with MmpL3.Future Med Chem. 2025 Jan;17(1):19-34. doi: 10.1080/17568919.2024.2444872. Epub 2024 Dec 25. Future Med Chem. 2025. PMID: 39720921
References
-
- WHO | Tuberculosis (TB). WHO [Internet]. World Health Organization; 2018. [cited 2019 Mar 1]; Available from: https://www.who.int/gho/tb/en/
-
- World Health Organization. BCG vaccine: WHO position paper, February 2018 – Recommendations. Vaccine. 2018. - PubMed
-
- WHO. Guidelines for treatment of drug-susceptible tuberculosis and patient care. World Health Organization. 2017.
-
- Rožman K, Sosič I, Fernandez R, Young RJ, Mendoza A, Gobec S, et al. A new ‘golden age’ for the antitubercular target InhA. Drug Discovery Today. 2017. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

