Regioselective Glycosylation Strategies for the Synthesis of Group Ia and Ib Streptococcus Related Glycans Enable Elucidating Unique Conformations of the Capsular Polysaccharides
- PMID: 31506992
- PMCID: PMC6972993
- DOI: 10.1002/chem.201903527
Regioselective Glycosylation Strategies for the Synthesis of Group Ia and Ib Streptococcus Related Glycans Enable Elucidating Unique Conformations of the Capsular Polysaccharides
Abstract
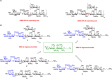
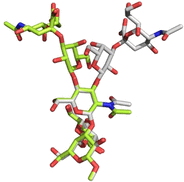
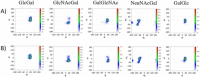
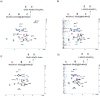
Group B Streptococcus serotypes Ia and Ib capsular polysaccharides are key targets for vaccine development. In spite of their immunospecifity these polysaccharides share high structural similarity. Both are composed of the same monosaccharide residues and differ only in the connection of the Neu5Acα2-3Gal side chain to the GlcNAc unit, which is a β1-4 linkage in serotype Ia and a β1-3 linkage in serotype Ib. The development of efficient regioselective routes for GlcNAcβ1-3[Glcβ1-4]Gal synthons is described, which give access to different group B Streptococcus (GBS) Ia and Ib repeating unit frameshifts. These glycans were used to probe the conformation and molecular dynamics of the two polysaccharides, highlighting the different presentation of the protruding Neu5Acα2-3Gal moieties on the polysaccharide backbones and a higher flexibility of Ib polymer relative to Ia, which can impact epitope exposure.
Keywords: carbohydrates; conformation analysis; glycosylation; regioselectivity; therapeutics.
© 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
L.D.B., D.O., M.M.R., R.C., and R.A. are employees of GSK groups companies. L.D.B. and R.A. are inventors of a patent related to the topic. R.A. is owner of GSK stocks.
Figures







Similar articles
-
Chemical synthesis of the dimeric repeating unit of type Ia group B Streptococcus capsular polysaccharide.Org Biomol Chem. 2019 Jun 12;17(23):5839-5848. doi: 10.1039/c9ob01024f. Org Biomol Chem. 2019. PMID: 31157343
-
Chemical synthesis of the repeating unit of type Ia group B Streptococcus capsular polysaccharide.Org Lett. 2015 Mar 6;17(5):1102-5. doi: 10.1021/ol5036563. Epub 2015 Feb 12. Org Lett. 2015. PMID: 25674920 Free PMC article.
-
Chemical Synthesis of the Repeating Unit of Type II Group B Streptococcus Capsular Polysaccharide.J Org Chem. 2018 Jun 1;83(11):5920-5930. doi: 10.1021/acs.joc.8b00396. Epub 2018 May 10. J Org Chem. 2018. PMID: 29709182
-
Exploring the Group B Streptococcus capsular polysaccharides: the structural diversity provides the basis for development of NMR-based identity assays.J Pharm Biomed Anal. 2014 Sep;98:9-15. doi: 10.1016/j.jpba.2014.05.004. Epub 2014 May 13. J Pharm Biomed Anal. 2014. PMID: 24873733 Review.
-
Staphylococcus aureus capsular polysaccharides: a structural and synthetic perspective.Org Biomol Chem. 2020 Feb 7;18(5):783-798. doi: 10.1039/c9ob02546d. Epub 2020 Jan 10. Org Biomol Chem. 2020. PMID: 31922180 Review.
Cited by
-
Borane-Trimethylamine Complex: A Versatile Reagent in Organic Synthesis.Molecules. 2024 Apr 27;29(9):2017. doi: 10.3390/molecules29092017. Molecules. 2024. PMID: 38731507 Free PMC article. Review.
-
Stereoselective Shi-type epoxidation with 3-oxo-4,6-O-benzylidene pyranoside catalysts: unveiling the role of carbohydrate skeletons.RSC Adv. 2024 May 23;14(24):16778-16783. doi: 10.1039/d4ra03011g. eCollection 2024 May 22. RSC Adv. 2024. PMID: 38784424 Free PMC article.
-
Calibration of a serum reference standard for Group B streptococcal polysaccharide conjugate vaccine development using surface plasmon resonance.NPJ Vaccines. 2023 May 19;8(1):71. doi: 10.1038/s41541-023-00667-1. NPJ Vaccines. 2023. PMID: 37208375 Free PMC article.
-
Conformational Studies of Oligosaccharides.Chemistry. 2020 Aug 6;26(44):9814-9825. doi: 10.1002/chem.202001370. Epub 2020 Jul 9. Chemistry. 2020. PMID: 32329095 Free PMC article. Review.
-
Structure-Guided Design of a Group B Streptococcus Type III Synthetic Glycan-Conjugate Vaccine.Chemistry. 2020 Jun 2;26(31):7018-7025. doi: 10.1002/chem.202000284. Epub 2020 Apr 1. Chemistry. 2020. PMID: 32058627 Free PMC article.
References
-
- None
-
- Paoletti L. C., Kasper D. L., Expert Opin. Biol. Ther. 2003, 3, 975–984; - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

