A two-stage phase II clinical trial design with nested criteria for early stopping and efficacy
- PMID: 31507079
- PMCID: PMC6996237
- DOI: 10.1002/pst.1965
A two-stage phase II clinical trial design with nested criteria for early stopping and efficacy
Abstract
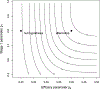
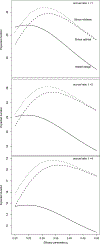
We propose a two-stage design for a single arm clinical trial with an early stopping rule for futility. This design employs different endpoints to assess early stopping and efficacy. The early stopping rule is based on a criteria determined more quickly than that for efficacy. These separate criteria are also nested in the sense that efficacy is a special case of, but usually not identical to, the early stopping endpoint. The design readily allows for planning in terms of statistical significance, power, expected sample size, and expected duration. This method is illustrated with a phase II design comparing rates of disease progression in elderly patients treated for lung cancer to rates found using a historical control. In this example, the early stopping rule is based on the number of patients who exhibit progression-free survival (PFS) at 2 months post treatment follow-up. Efficacy is judged by the number of patients who have PFS at 6 months. We demonstrate our design has expected sample size and power comparable with the Simon two-stage design but exhibits shorter expected duration under a range of useful parameter values.
Keywords: curtailed sampling; expected sample size; expected trial duration; minimax design.
© 2019 John Wiley & Sons, Ltd.
Figures

Similar articles
-
Optimal and minimax three-stage designs for phase II oncology clinical trials.Contemp Clin Trials. 2008 Jan;29(1):32-41. doi: 10.1016/j.cct.2007.04.008. Epub 2007 May 6. Contemp Clin Trials. 2008. PMID: 17544337
-
Two-stage phase II oncology designs using short-term endpoints for early stopping.Stat Methods Med Res. 2017 Aug;26(4):1671-1683. doi: 10.1177/0962280215585819. Epub 2015 Jun 2. Stat Methods Med Res. 2017. PMID: 26037529
-
A flexible multi-stage design for phase II oncology trials.Pharm Stat. 2011 Jul-Aug;10(4):369-73. doi: 10.1002/pst.478. Epub 2010 Dec 8. Pharm Stat. 2011. PMID: 22328328
-
Group-sequential methods for adaptive seamless phase II/III clinical trials.J Biopharm Stat. 2011 Jul;21(4):787-801. doi: 10.1080/10543406.2011.551335. J Biopharm Stat. 2011. PMID: 21516569 Review.
-
Statistical issues for design and analysis of single-arm multi-stage phase II cancer clinical trials.Contemp Clin Trials. 2015 May;42:9-17. doi: 10.1016/j.cct.2015.02.007. Epub 2015 Mar 3. Contemp Clin Trials. 2015. PMID: 25749311 Free PMC article. Review.
Cited by
-
Using short-term endpoints to improve interim decision making and trial duration in two-stage phase II trials with nested binary endpoints.Stat Methods Med Res. 2023 Sep;32(9):1749-1765. doi: 10.1177/09622802231188515. Epub 2023 Jul 25. Stat Methods Med Res. 2023. PMID: 37489267 Free PMC article.
-
Bayesian single-to-double arm transition design using both short-term and long-term endpoints.Pharm Stat. 2023 Jul-Aug;22(4):588-604. doi: 10.1002/pst.2292. Epub 2023 Feb 8. Pharm Stat. 2023. PMID: 36755420 Free PMC article.
References
-
- Simon R. Optimal two-stage designs for. Controlled Clinical Trials. 1989;10(1):1–10. - PubMed
-
- De With C.. Two-stage plans for the testing of binomial parameters. Contemp Clin Trials. 1983;4(3):215–26. - PubMed
-
- Hanfelt JJ, Slack RS, Gehan EA. A modification of simon’s optimal design for phase ii trials when the criterion is median sample size. Control Clin Trials. 1999;20(6):555–66. - PubMed
-
- Jung SH, Carey M, Kim KM. Graphical search for two-stage designs for phase ii clinical trials. Control Clin Trials. 2001;22(4):367–72. - PubMed
-
- Korn EL, Freidlin B. Conditional power calculations for clinical trials with historical controls. Statistics in Medicine. 2006;25(17):2922–31. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

