Perspectives on the Barrier to Resistance for Dolutegravir + Lamivudine, a Two-Drug Antiretroviral Therapy for HIV-1 Infection
- PMID: 31507204
- PMCID: PMC6944139
- DOI: 10.1089/AID.2019.0171
Perspectives on the Barrier to Resistance for Dolutegravir + Lamivudine, a Two-Drug Antiretroviral Therapy for HIV-1 Infection
Abstract
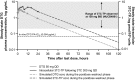
In HIV-1-infected patients, virological failure can occur as a consequence of the mutations that accumulate in the viral genome that allow replication to continue in the presence of antiretrovirals (ARVs). The development of treatment-emergent resistance to an ARV can limit a patient's options for future therapy, prompting the need for ARV regimens that are resilient to the emergence of resistance. The genetic barrier to resistance refers to the number of mutations in an ARV's therapeutic target that are required to confer a clinically meaningful loss of susceptibility to the drug. The emergence of resistance can be affected by pharmacological aspects of the ARV, including its structure, inhibitory quotient, therapeutic index, and pharmacokinetic characteristics. Dolutegravir (DTG) has demonstrated a high barrier to resistance, including when used in a two-drug regimen (2DR) with lamivudine (3TC). In the GEMINI-1 and GEMINI-2 studies, DTG +3TC was noninferior to DTG + emtricitabine/tenofovir disoproxil fumarate in treatment-naive participants, with similar proportions achieving HIV-1 RNA <50 copies/mL through 96 weeks. Furthermore, in the TANGO study, virological suppression was maintained at 48 weeks after switching to DTG +3TC from a tenofovir alafenamide (TAF)-based regimen compared with continuing a TAF-based regimen. Most other 2DRs with successful outcomes compared with three-drug regimens have been based on protease inhibitors (PIs); however, this class is associated with adverse metabolic effects and drug-drug interactions. In this review, we discuss the barrier to resistance in the context of a 2DR in which a boosted PI is replaced with DTG +3TC.
Keywords: HIV; antiretroviral; genetic barrier to resistance; integrase strand transfer inhibitor; two-drug regimen.
Conflict of interest statement
M.B. has received speaking and advising fees from Gilead, ViiV Healthcare, Merck Sharp & Dohme, and Janssen; traveling grants from Gilead; and research grants to the institution from Gilead, ViiV Healthcare, Merck Sharp & Dohme, Janssen, Bristol-Myers Squibb, and Mylan. L.W. reports personal fees from ViiV Healthcare, Gilead, Merck Sharp & Dohme, Janssen, and Mylan outside the submitted study; L.W. is a member of the HIV Clinical Reference Group, advising NHS England on HIV treatment and care; L.W. cochairs the drugs subgroup; L.W. chairs the British HIV Association ART guidelines. P.C. reports grants from AbbVie, advisory board fees from Merck Sharp & Dohme, and grants and advisory board fees from ViiV Healthcare outside the submitted study. R.P. reports grants and consulting fees from ViiV Healthcare, Merck Sharp & Dohme, and Gilead during the conduct of the study. J.K., J.V.W., T.V., K.A., and R.Q. are employees of ViiV Healthcare and own stock in GlaxoSmithKline. J.D. was an employee of ViiV Healthcare during the time of the study and owns stock/stock options in GlaxoSmithKline.
Figures
Comment in
-
Prior Case of Resistance on Dolutegravir Plus Lamivudine Dual Therapy.AIDS Res Hum Retroviruses. 2020 Apr;36(4):254-255. doi: 10.1089/AID.2019.0253. Epub 2020 Mar 9. AIDS Res Hum Retroviruses. 2020. PMID: 31914799 Free PMC article. No abstract available.
References
-
- US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER): Guidance for industry: Role of HIV resistance testing in antiretroviral drug development. Available at https://fda.gov/regulatory-information/search-fda-guidance-documents/rol... (2007), accessed June24, 2019
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
