Promoting vaccination in maternity wards ─ motivational interview technique reduces hesitancy and enhances intention to vaccinate, results from a multicentre non-controlled pre- and post-intervention RCT-nested study, Quebec, March 2014 to February 2015
- PMID: 31507265
- PMCID: PMC6737828
- DOI: 10.2807/1560-7917.ES.2019.24.36.1800641
Promoting vaccination in maternity wards ─ motivational interview technique reduces hesitancy and enhances intention to vaccinate, results from a multicentre non-controlled pre- and post-intervention RCT-nested study, Quebec, March 2014 to February 2015
Abstract
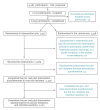
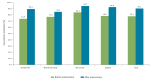
BackgroundMany countries are grappling with growing numbers of parents who delay or refuse recommended vaccinations for their children. This has created a need for strategies to address vaccine hesitancy (VH) and better support parental decision-making regarding vaccination.AimTo assess vaccination intention (VI) and VH among parents who received an individual motivational-interview (MI) based intervention on infant immunisation during post-partum stay at a maternity ward between March 2014 and February 2015.MethodsThis non-controlled pre-/post-intervention study was conducted using the results from parents enrolled in the intervention arm of the PromoVaQ randomised control trial (RCT), which was conducted in four maternity wards across the Province of Quebec. Participants (n = 1,223) completed pre- and post-intervention questionnaires on VI and VH using Opel's score. Pre-/post-intervention measures were compared using McNemar's test for categorical variables and Wilcoxon signed-rank test for continuous variables.ResultsPre-intervention: overall VI was 78% and significantly differed across maternity wards (74%, 77%, 84%, 79%, p = 0.02). Post-intervention: VI rose significantly across maternity wards (89%, 85%, 95%, 93%) and the overall increase in VI was 12% (78% vs 90%, p < 0.0001). VH corroborated these observations, pre- vs post-intervention, for each maternity ward (28% vs 16%, 29% vs 21%, 27% vs 17%, 24% vs 13%). Overall, VH was curbed post-intervention by 40% (27% vs 16%; p < 0.0001).ConclusionsCompared with pre-intervention status, participants who received the MI-based intervention on immunisation displayed lower hesitancy and greater intention to vaccinate their infant at 2 months of age.
Keywords: Canada; hesitancy; immunisation; infant; intention; motivational interviewing; parents; vaccine; vaccines.
Conflict of interest statement
Figures
References
-
- World Health Organization (WHO). New assessment report on progress towards global immunization goals. Geneva: WHO; 2017. Available from: http://wwwwhoint/immunization/global_vaccine_action_plan/sage_gvap_octob...
-
- World Health Organization (WHO). Progress and challenges with achieving universal immunization coverage. Geneva: WHO; [accessed Jul 2018]. Available from: https://www.who.int/immunization/monitoring_surveillance/who-immuniz.pdf.
-
- Institut national de santé publique du Québec (INSPQ). Enquête sur la couverture vaccinale des enfants de 1 an et 2 ans au Québec en 2016 [Survey of vaccine coverage of 1-year and 2-year old children in Quebec in 2016]. Québec: INSPQ; 2017. Available from: https://www.inspq.qc.ca/sites/default/files/publications/2341_enquete_co....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
