Vagus Nerve Stimulation-Induced Laryngeal Motor Evoked Potentials: A Possible Biomarker of Effective Nerve Activation
- PMID: 31507360
- PMCID: PMC6718640
- DOI: 10.3389/fnins.2019.00880
Vagus Nerve Stimulation-Induced Laryngeal Motor Evoked Potentials: A Possible Biomarker of Effective Nerve Activation
Abstract
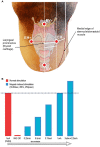
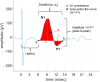
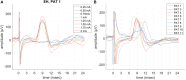
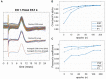
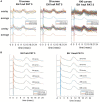
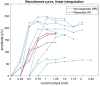
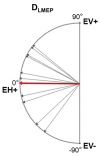
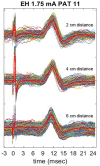
Vagus nerve stimulation (VNS) therapy is associated with laryngeal muscle activation and induces voice modifications, well-known side effects of the therapy resulting from co-activation of the recurrent laryngeal nerve. In this study, we describe the non-invasive transcutaneous recording of laryngeal motor evoked potentials (LMEPs), which could serve as a biomarker of effective nerve activation and individual titration in patients with drug-resistant epilepsy. We recruited drug-resistant epileptic patients treated for at least 6 months with a VNS. Trains of 600-1200 VNS pulses were delivered with increasing current outputs. We placed six skin electrodes on the ventral surface of the neck, in order to record LMEPs whenever the laryngeal muscular threshold was reached. We studied the internal consistency and the variability of LMEP recordings, and compared different methods for amplitude calculation. Recruitment curves were built based on the stimulus-response relationship. We also determined the electrical axis of the LMEPs dipole in order to define the optimal electrode placement for LMEPs recording in a clinical setting. LMEPs were successfully recorded in 11/11 patients. The LMEPs threshold ranged from 0.25 to 1 mA (median 0.50 mA), and onset latency was between 5.37 and 8.77 ms. The signal-to-noise ratio was outstanding in 10/11 patients. In these cases, excellent reliability (Intraclass correlation coefficient, ICC > 0.90 across three different amplitude measurements) was achieved with 10 sample averages. Moreover, our recordings showed very good internal consistency (Cronbach's alpha > 0.95 for 10 epochs). Area-under-the-curve and peak-to-peak measurement proved to be complementary methods for amplitude calculation. Finally, we determined that an optimal derivation requires only two recording electrodes, aligned on a horizontal axis around the laryngeal prominence. In conclusion, we describe here an optimal methodology for the recording of VNS-induced motor evoked responses from the larynx. Although further clinical validation is still necessary, LMEPs might be useful as a non-invasive marker of effective nerve activation, and as an aid for the clinician to perform a more rational titration of VNS parameters.
Keywords: biomarkers; electrical axis; epilepsy; internal consistency; larynx; motor evoked potentials; reliability; vagus nerve stimulation (VNS).
Figures
Similar articles
-
A Preclinical Study of Laryngeal Motor-Evoked Potentials as a Marker Vagus Nerve Activation.Int J Neural Syst. 2015 Dec;25(8):1550034. doi: 10.1142/S0129065715500343. Epub 2015 Sep 14. Int J Neural Syst. 2015. PMID: 26510476
-
Laryngeal Muscle-Evoked Potential Recording as an Indicator of Vagal Nerve Fiber Activation.Neuromodulation. 2022 Apr;25(3):461-470. doi: 10.1016/j.neurom.2022.01.014. Epub 2022 Feb 15. Neuromodulation. 2022. PMID: 35177376
-
Vagus nerve stimulation-induced laryngeal motor evoked potentials for response prediction and intensity titration in drug-resistant epilepsy.Clin Neurophysiol. 2023 Mar;147:99-107. doi: 10.1016/j.clinph.2023.01.009. Epub 2023 Jan 27. Clin Neurophysiol. 2023. PMID: 36764043
-
Vagus nerve stimulation: Surgical technique of implantation and revision and related morbidity.Epilepsia. 2017 Apr;58 Suppl 1:85-90. doi: 10.1111/epi.13678. Epilepsia. 2017. PMID: 28386925 Review.
-
Research progress on the electrophysiological indicators to predict the efficacy of vagus nerve stimulation for drug-refractory epilepsy.Acta Epileptol. 2024 Mar 1;6(1):7. doi: 10.1186/s42494-023-00147-y. Acta Epileptol. 2024. PMID: 40217388 Free PMC article. Review.
Cited by
-
Closed-Loop Vagus Nerve Stimulation for the Treatment of Cardiovascular Diseases: State of the Art and Future Directions.Front Cardiovasc Med. 2022 Apr 7;9:866957. doi: 10.3389/fcvm.2022.866957. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35463766 Free PMC article. Review.
-
Using neural biomarkers to personalize dosing of vagus nerve stimulation.Bioelectron Med. 2024 Jun 17;10(1):15. doi: 10.1186/s42234-024-00147-4. Bioelectron Med. 2024. PMID: 38880906 Free PMC article.
-
Vagal nerve magnetic stimulation for post-traumatic cricopharyngeal achalasia with bilateral vocal cord paralysis: A case report.Medicine (Baltimore). 2025 Jul 25;104(30):e43525. doi: 10.1097/MD.0000000000043525. Medicine (Baltimore). 2025. PMID: 40725869 Free PMC article.
-
Bioelectronic Therapeutics: A Revolutionary Medical Practice in Health Care.Bioelectricity. 2025 Mar 18;7(1):2-28. doi: 10.1089/bioe.2024.0039. eCollection 2025 Mar. Bioelectricity. 2025. PMID: 40342937 Review.
-
Laterality, sexual dimorphism, and human vagal projectome heterogeneity shape neuromodulation to vagus nerve stimulation.Commun Biol. 2024 Nov 19;7(1):1536. doi: 10.1038/s42003-024-07222-1. Commun Biol. 2024. PMID: 39562711 Free PMC article.
References
-
- Ben-Menachem E., Manon-Espaillat R., Ristanovic R., Wilder B. J., Stefan H., Mirza W., et al. (1994). Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. First international vagus nerve stimulation study group. Epilepsia 35 616–626. 10.1111/j.1528-1157.1994.tb02482.x - DOI - PubMed
LinkOut - more resources
Full Text Sources

