Secretin Regulates Excitatory GABAergic Neurotransmission to GnRH Neurons via Retrograde NO Signaling Pathway in Mice
- PMID: 31507377
- PMCID: PMC6716020
- DOI: 10.3389/fncel.2019.00371
Secretin Regulates Excitatory GABAergic Neurotransmission to GnRH Neurons via Retrograde NO Signaling Pathway in Mice
Abstract
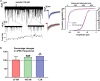
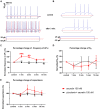
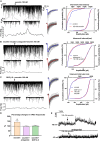
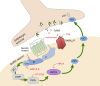
In mammals, reproduction is regulated by a wide range of metabolic hormones that maintain the proper energy balance. In addition to regulating feeding and energy expenditure, these metabolic messengers also modulate the functional performance of the hypothalamic-pituitary-gonadal (HPG) axis. Secretin, a member of the secretin-glucagon-vasoactive intestinal peptide hormone family, has been shown to alter reproduction centrally, although the underlying mechanisms have not been explored yet. In order to elucidate its central action in the neuroendocrine regulation of reproduction, in vitro electrophysiological slice experiments were carried out on GnRH-GFP neurons in male mice. Bath application of secretin (100 nM) significantly increased the frequency of the spontaneous postsynaptic currents (sPSCs) to 118.0 ± 2.64% compared to the control, and that of the GABAergic miniature postsynaptic currents (mPSCs) to 147.6 ± 19.19%. Resting membrane potential became depolarized by 12.74 ± 4.539 mV after secretin treatment. Frequency of evoked action potentials (APs) also increased to 144.3 ± 10.8%. The secretin-triggered elevation of the frequency of mPSCs was prevented by using either a secretin receptor antagonist (3 μM) or intracellularly applied G-protein-coupled receptor blocker (GDP-β-S; 2 mM) supporting the involvement of secretin receptor in the process. Regarding the actions downstream to secretin receptor, intracellular blockade of protein kinase A (PKA) with KT-5720 (2 μM) or intracellular inhibition of the neuronal nitric oxide synthase (nNOS) by NPLA (1 μM) abolished the stimulatory effect of secretin on mPSCs. These data suggest that secretin acts on GnRH neurons via secretin receptors whose activation triggers the cAMP/PKA/nNOS signaling pathway resulting in nitric oxide release and in the presynaptic terminals this retrograde NO machinery regulates the GABAergic input to GnRH neurons.
Keywords: GABA; GnRH neuron; metabolism; nitric oxide; reproduction; retrograde signaling; secretin.
Figures

Similar articles
-
Glucagon-Like Peptide-1 Excites Firing and Increases GABAergic Miniature Postsynaptic Currents (mPSCs) in Gonadotropin-Releasing Hormone (GnRH) Neurons of the Male Mice via Activation of Nitric Oxide (NO) and Suppression of Endocannabinoid Signaling Pathways.Front Cell Neurosci. 2016 Sep 12;10:214. doi: 10.3389/fncel.2016.00214. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27672360 Free PMC article.
-
Insulin-Like Growth Factor 1 Increases GABAergic Neurotransmission to GnRH Neurons via Suppressing the Retrograde Tonic Endocannabinoid Signaling Pathway in Mice.Neuroendocrinology. 2021;111(12):1219-1230. doi: 10.1159/000514043. Epub 2020 Dec 24. Neuroendocrinology. 2021. PMID: 33361699
-
Estrogen Receptor Beta and 2-arachidonoylglycerol Mediate the Suppressive Effects of Estradiol on Frequency of Postsynaptic Currents in Gonadotropin-Releasing Hormone Neurons of Metestrous Mice: An Acute Slice Electrophysiological Study.Front Cell Neurosci. 2016 Mar 29;10:77. doi: 10.3389/fncel.2016.00077. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27065803 Free PMC article.
-
GABAergic regulation of the HPA and HPG axes and the impact of stress on reproductive function.J Steroid Biochem Mol Biol. 2016 Jun;160:196-203. doi: 10.1016/j.jsbmb.2015.11.019. Epub 2015 Dec 9. J Steroid Biochem Mol Biol. 2016. PMID: 26690789 Free PMC article. Review.
-
Secretin, glucagon, gastric inhibitory polypeptide, parathyroid hormone, and related peptides in the regulation of the hypothalamus- pituitary-adrenal axis.Peptides. 2000 Feb;21(2):309-24. doi: 10.1016/s0196-9781(99)00193-x. Peptides. 2000. PMID: 10764961 Review.
Cited by
-
Social boldness correlates with brain gene expression in male green anoles.Horm Behav. 2021 Jul;133:105007. doi: 10.1016/j.yhbeh.2021.105007. Epub 2021 Jun 5. Horm Behav. 2021. PMID: 34102460 Free PMC article.
-
Genome-wide single nucleotide polymorphism (SNP) data reveal potential candidate genes for litter traits in a Yorkshire pig population.Arch Anim Breed. 2023 Nov 23;66(4):357-368. doi: 10.5194/aab-66-357-2023. eCollection 2023. Arch Anim Breed. 2023. PMID: 38111388 Free PMC article.
-
Gonadal Cycle-Dependent Expression of Genes Encoding Peptide-, Growth Factor-, and Orphan G-Protein-Coupled Receptors in Gonadotropin- Releasing Hormone Neurons of Mice.Front Mol Neurosci. 2021 Jan 18;13:594119. doi: 10.3389/fnmol.2020.594119. eCollection 2020. Front Mol Neurosci. 2021. PMID: 33551743 Free PMC article.
-
The electrophysiologic properties of gonadotropin-releasing hormone neurons.J Neuroendocrinol. 2022 May;34(5):e13073. doi: 10.1111/jne.13073. Epub 2021 Dec 22. J Neuroendocrinol. 2022. PMID: 34939256 Free PMC article. Review.
-
Cholinergic Control of GnRH Neuron Physiology and Luteinizing Hormone Secretion in Male Mice: Involvement of ACh/GABA Cotransmission.J Neurosci. 2024 Mar 20;44(12):e1780232024. doi: 10.1523/JNEUROSCI.1780-23.2024. J Neurosci. 2024. PMID: 38320853 Free PMC article.
References
-
- Barbour B. (2014). Electronics for Electrophysiologists. Available at: http://www.biologie.ens.fr/~barbour/electronics_for_electrophysiologists...
LinkOut - more resources
Full Text Sources
Miscellaneous

