The Concept of Transmission Coefficient Among Different Cerebellar Layers: A Computational Tool for Analyzing Motor Learning
- PMID: 31507382
- PMCID: PMC6718712
- DOI: 10.3389/fncir.2019.00054
The Concept of Transmission Coefficient Among Different Cerebellar Layers: A Computational Tool for Analyzing Motor Learning
Abstract
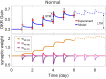
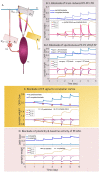
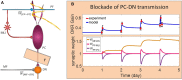
High-fidelity regulation of information transmission among cerebellar layers is mainly provided by synaptic plasticity. Therefore, determining the regulatory foundations of synaptic plasticity in the cerebellum and translating them to behavioral output are of great importance. To date, many experimental studies have been carried out in order to clarify the effect of synaptic defects, while targeting a specific signaling pathway in the cerebellar function. However, the contradictory results of these studies at the behavioral level further add to the ambiguity of the problem. Information transmission through firing rate changes in populations of interconnected neurons is one of the most widely accepted principles of neural coding. In this study, while considering the efficacy of synaptic interactions among the cerebellar layers, we propose a firing rate model to realize the concept of transmission coefficient. Thereafter, using a computational approach, we test the effect of different values of transmission coefficient on the gain adaptation of a cerebellar-dependent motor learning task. In conformity with the behavioral data, the proposed model can accurately predict that disruption in different forms of synaptic plasticity does not have the same effect on motor learning. Specifically, impairment in training mechanisms, like in the train-induced LTD in parallel fiber-Purkinje cell synapses, has a significant negative impact on all aspects of learning, including memory formation, transfer, and consolidation, although it does not disrupt basic motor performance. In this regard, the overinduction of parallel fiber-molecular layer interneuron LTP could not prevent motor learning impairment, despite its vital role in preserving the robustness of basic motor performance. In contrast, impairment in plasticity induced by interneurons and background activity of climbing fibers is partly compensable through overinduction of train-induced parallel fiber-Purkinje cell LTD. Additionally, blockade of climbing fiber signaling to the cerebellar cortex, referred to as olivary system lesion, shows the most destructive effect on both motor learning and basic motor performance. Overall, the obtained results from the proposed computational framework are used to provide a map from procedural motor memory formation in the cerebellum. Certainly, the generalization of this concept to other multi-layered networks of the brain requires more physiological and computational researches.
Keywords: cerebellar motor learning; multi-layer neural network; optokinetic reflex; synaptic plasticity; transmission coefficient.
Figures




Similar articles
-
Computational Theory Underlying Acute Vestibulo-ocular Reflex Motor Learning with Cerebellar Long-Term Depression and Long-Term Potentiation.Cerebellum. 2017 Aug;16(4):827-839. doi: 10.1007/s12311-017-0857-6. Cerebellum. 2017. PMID: 28444617
-
Long-Term Depression of Intrinsic Excitability Accompanied by Synaptic Depression in Cerebellar Purkinje Cells.J Neurosci. 2017 Jun 7;37(23):5659-5669. doi: 10.1523/JNEUROSCI.3464-16.2017. Epub 2017 May 11. J Neurosci. 2017. PMID: 28495974 Free PMC article.
-
Synaptic memories upside down: bidirectional plasticity at cerebellar parallel fiber-Purkinje cell synapses.Neuron. 2006 Oct 19;52(2):227-38. doi: 10.1016/j.neuron.2006.09.032. Neuron. 2006. PMID: 17046686 Review.
-
The role of calcium in synaptic plasticity and motor learning in the cerebellar cortex.Neurosci Biobehav Rev. 2012 Apr;36(4):1153-62. doi: 10.1016/j.neubiorev.2012.01.005. Epub 2012 Jan 28. Neurosci Biobehav Rev. 2012. PMID: 22305995 Review.
-
Estradiol Enhances Cerebellar Molecular Layer Interneuron-Purkinje Cell Synaptic Transmission and Improves Motor Learning Through ER-β in Vivo in Mice.Cerebellum. 2025 Feb 21;24(2):51. doi: 10.1007/s12311-025-01805-2. Cerebellum. 2025. PMID: 39979512
Cited by
-
Cerebellar Micro Complex Model Using Histologic Boolean Mapping Simulates Adaptive Motor Control.Neuroinformatics. 2025 Jun 17;23(3):35. doi: 10.1007/s12021-025-09730-9. Neuroinformatics. 2025. PMID: 40526235 Free PMC article.
-
Repetitive transcranial magnetic stimulation promotes motor function recovery in mice after spinal cord injury via regulation of the Cx43-autophagy loop.J Orthop Surg Res. 2024 Jul 2;19(1):387. doi: 10.1186/s13018-024-04879-6. J Orthop Surg Res. 2024. PMID: 38956661 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources

