Interrogating Synaptic Architecture: Approaches for Labeling Organelles and Cytoskeleton Components
- PMID: 31507402
- PMCID: PMC6716447
- DOI: 10.3389/fnsyn.2019.00023
Interrogating Synaptic Architecture: Approaches for Labeling Organelles and Cytoskeleton Components
Abstract
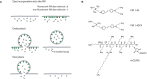
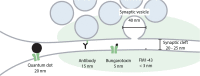
Synaptic transmission has been studied for decades, as a fundamental step in brain function. The structure of the synapse, and its changes during activity, turned out to be key aspects not only in the transfer of information between neurons, but also in cognitive processes such as learning and memory. The overall synaptic morphology has traditionally been studied by electron microscopy, which enables the visualization of synaptic structure in great detail. The changes in the organization of easily identified structures, such as the presynaptic active zone, or the postsynaptic density, are optimally studied via electron microscopy. However, few reliable methods are available for labeling individual organelles or protein complexes in electron microscopy. For such targets one typically relies either on combination of electron and fluorescence microscopy, or on super-resolution fluorescence microscopy. This review focuses on approaches and techniques used to specifically reveal synaptic organelles and protein complexes, such as cytoskeletal assemblies. We place the strongest emphasis on methods detecting the targets of interest by affinity binding, and we discuss the advantages and limitations of each method.
Keywords: actin; cytoskeleton; nanoscopy; super-resolution; synapse; vesicles.
Figures



Similar articles
-
Towards a Comprehensive Optical Connectome at Single Synapse Resolution via Expansion Microscopy.Front Synaptic Neurosci. 2022 Jan 18;13:754814. doi: 10.3389/fnsyn.2021.754814. eCollection 2021. Front Synaptic Neurosci. 2022. PMID: 35115916 Free PMC article. Review.
-
The cytoskeletal architecture of the presynaptic terminal and molecular structure of synapsin 1.J Cell Biol. 1989 Jan;108(1):111-26. doi: 10.1083/jcb.108.1.111. J Cell Biol. 1989. PMID: 2536030 Free PMC article.
-
Enhanced synaptic protein visualization by multicolor super-resolution expansion microscopy.Neurophotonics. 2023 Oct;10(4):044412. doi: 10.1117/1.NPh.10.4.044412. Epub 2023 Oct 25. Neurophotonics. 2023. PMID: 37886043 Free PMC article.
-
Postsynaptic actin regulates active zone spacing and glutamate receptor apposition at the Drosophila neuromuscular junction.Mol Cell Neurosci. 2014 Jul;61:241-54. doi: 10.1016/j.mcn.2014.07.005. Epub 2014 Jul 24. Mol Cell Neurosci. 2014. PMID: 25066865 Free PMC article.
-
Relevance of presynaptic actin dynamics for synapse function and mouse behavior.Exp Cell Res. 2015 Jul 15;335(2):165-71. doi: 10.1016/j.yexcr.2014.12.020. Epub 2015 Jan 8. Exp Cell Res. 2015. PMID: 25579398 Review.
Cited by
-
Presynapses contain distinct actin nanostructures.J Cell Biol. 2023 Oct 2;222(10):e202208110. doi: 10.1083/jcb.202208110. Epub 2023 Aug 14. J Cell Biol. 2023. PMID: 37578754 Free PMC article.
-
Gephyrin-Lacking PV Synapses on Neocortical Pyramidal Neurons.Int J Mol Sci. 2021 Sep 17;22(18):10032. doi: 10.3390/ijms221810032. Int J Mol Sci. 2021. PMID: 34576197 Free PMC article.
-
Microglial Pruning: Relevance for Synaptic Dysfunction in Multiple Sclerosis and Related Experimental Models.Cells. 2021 Mar 20;10(3):686. doi: 10.3390/cells10030686. Cells. 2021. PMID: 33804596 Free PMC article. Review.
-
Role of actin cytoskeleton in the organization and function of ionotropic glutamate receptors.Curr Res Struct Biol. 2021 Oct 14;3:277-289. doi: 10.1016/j.crstbi.2021.10.001. eCollection 2021. Curr Res Struct Biol. 2021. PMID: 34766008 Free PMC article. Review.
-
Super-resolution STED imaging in the inner and outer whole-mount mouse retina.Front Ophthalmol (Lausanne). 2023 Apr 6;3:1126338. doi: 10.3389/fopht.2023.1126338. eCollection 2023. Front Ophthalmol (Lausanne). 2023. PMID: 38983015 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

