Synergistic Effects of Curcumin and Piperine as Potent Acetylcholine and Amyloidogenic Inhibitors With Significant Neuroprotective Activity in SH-SY5Y Cells via Computational Molecular Modeling and in vitro Assay
- PMID: 31507403
- PMCID: PMC6718453
- DOI: 10.3389/fnagi.2019.00206
Synergistic Effects of Curcumin and Piperine as Potent Acetylcholine and Amyloidogenic Inhibitors With Significant Neuroprotective Activity in SH-SY5Y Cells via Computational Molecular Modeling and in vitro Assay
Abstract
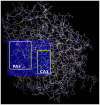
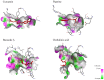
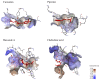
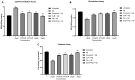
Hallmarks of Alzheimer's disease (AD) pathology include acetylcholine (ACh) deficiency and plaque deposition. Emerging studies suggest that acetylcholinesterase (AChE) may interact with amyloid β (Aβ) to promote aggregation of insoluble Aβ plaques in brains of patients. Current therapeutic options available for AD patients, such as AChE inhibitors, provide only symptomatic relief. In this study, we screened four natural compounds believed to harbor cognitive benefits-curcumin, piperine, bacoside A, and chebulinic acid. In the first section, preliminary screening through computational molecular docking simulations gauged the suitability of the compounds as novel AChE inhibitors. From here, only compounds that met the in silico selection criteria were selected for the second section through in vitro investigations, including AChE enzyme inhibition assay, 3-(4,5-dimenthylthiazol-2-yl)-2,5-dimethyltetrazolium bromide (MTT) assay, Thioflavin T (ThT) assay, and biochemical analysis via a neuronal cell line model. Of the four compounds screened, only curcumin (-9.6 kcal/mol) and piperine (-10.5 kcal/mol) showed favorable binding affinities and interactions towards AChE and were hence selected. In vitro AChE inhibition demonstrated that combination of curcumin and piperine showed greater AChE inhibition with an IC50 of 62.81 ± 0.01 μg/ml as compared to individual compounds, i.e., IC50 of curcumin at 134.5 ± 0.06 μg/ml and IC50 of piperine at 76.6 ± 0.08 μg/ml. In the SH-SY5Y cell model, this combination preserved cell viability up to 85%, indicating that the compounds protect against Aβ-induced neuronal damage (p < 0.01). Interestingly, our results also showed that curcumin and piperine achieved a synergistic effect at 35 μM with an synergism quotient (SQ) value of 1.824. Synergistic behavior indicates that the combination of these two compounds at lower concentrations may provide a better outcome than singularly used for Aβ proteins. Combined curcumin and piperine managed to inhibit aggregation (reduced ThT intensity at 0.432 a.u.; p < 0.01) as well as disaggregation (reduced ThT intensity at 0.532 a.u.; p < 0.01) of fibrillar Aβ42. Furthermore, combined curcumin and piperine reversed the Aβ-induced up-regulation of neuronal oxidative stress (p < 0.01). In conclusion, curcumin and piperine demonstrated promising neuroprotective effects, whereas bacoside A and chebulinic acid may not be suitable lead compounds. These results are hoped to advance the field of natural products research as potentially therapeutic and curative AD agents.
Keywords: Alzheimer’s disease; acetylcholinesterase; amyloid beta; curcumin; piperine.
Figures







Similar articles
-
Explicating anti-amyloidogenic role of curcumin and piperine via amyloid beta (Aβ) explicit pathway: recovery and reversal paradigm effects.PeerJ. 2020 Sep 30;8:e10003. doi: 10.7717/peerj.10003. eCollection 2020. PeerJ. 2020. PMID: 33062432 Free PMC article.
-
6-Methyluracil derivatives as acetylcholinesterase inhibitors for treatment of Alzheimer's disease.Int J Risk Saf Med. 2015;27 Suppl 1:S69-71. doi: 10.3233/JRS-150694. Int J Risk Saf Med. 2015. PMID: 26639718
-
Click-designed vanilloid-triazole conjugates as dual inhibitors of AChE and Aβ aggregation.RSC Adv. 2023 Jan 19;13(5):2871-2883. doi: 10.1039/d2ra07539c. eCollection 2023 Jan 18. RSC Adv. 2023. PMID: 36756452 Free PMC article.
-
Targeting beta-amyloid pathogenesis through acetylcholinesterase inhibitors.Curr Pharm Des. 2006;12(33):4377-87. doi: 10.2174/138161206778792985. Curr Pharm Des. 2006. PMID: 17105433 Review.
-
Mechanistic Insight into the Design of Chemical Tools to Control Multiple Pathogenic Features in Alzheimer's Disease.Acc Chem Res. 2021 Oct 19;54(20):3930-3940. doi: 10.1021/acs.accounts.1c00457. Epub 2021 Oct 4. Acc Chem Res. 2021. PMID: 34606227 Review.
Cited by
-
Explicating anti-amyloidogenic role of curcumin and piperine via amyloid beta (Aβ) explicit pathway: recovery and reversal paradigm effects.PeerJ. 2020 Sep 30;8:e10003. doi: 10.7717/peerj.10003. eCollection 2020. PeerJ. 2020. PMID: 33062432 Free PMC article.
-
Unveiling Spanlastics as a Novel Carrier for Drug Delivery: A Review.Pharm Nanotechnol. 2025;13(1):133-142. doi: 10.2174/0122117385286921240103113543. Pharm Nanotechnol. 2025. PMID: 38258763 Review.
-
Magnetic Levitational Assembly of Differentiated SH-SY5Y Cells for Aβ-Induced 3D Alzheimer's Disease Modeling and Curcumin Screening.Macromol Biosci. 2025 Jun;25(6):e2400658. doi: 10.1002/mabi.202400658. Epub 2025 Mar 25. Macromol Biosci. 2025. PMID: 40130456 Free PMC article.
-
Neural regeneration research model to be explored: SH-SY5Y human neuroblastoma cells.Neural Regen Res. 2023 Jun;18(6):1265-1266. doi: 10.4103/1673-5374.358621. Neural Regen Res. 2023. PMID: 36453406 Free PMC article. No abstract available.
-
Amorphous Polymer-Phospholipid Solid Dispersions for the Co-Delivery of Curcumin and Piperine Prepared via Hot-Melt Extrusion.Pharmaceutics. 2024 Jul 28;16(8):999. doi: 10.3390/pharmaceutics16080999. Pharmaceutics. 2024. PMID: 39204344 Free PMC article.
References
-
- Ali T., Kim T., Rehman S. U., Khan M. S., Amin F. U., Khan M., et al. . (2018). Natural dietary supplementation of anthocyanins via PI3K/Akt/Nrf2/HO-1 pathways mitigate oxidative stress, neurodegeneration and memory impairment in a mouse model of Alzheimer’s disease. Mol. Neurobiol. 55, 6076–6093. 10.1007/s12035-017-0798-6 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

