Brain Amide Proton Transfer Imaging of Rat With Alzheimer's Disease Using Saturation With Frequency Alternating RF Irradiation Method
- PMID: 31507405
- PMCID: PMC6713910
- DOI: 10.3389/fnagi.2019.00217
Brain Amide Proton Transfer Imaging of Rat With Alzheimer's Disease Using Saturation With Frequency Alternating RF Irradiation Method
Abstract
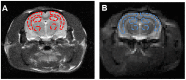
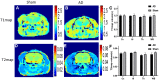
Amyloid-β (Aβ) deposits and some proteins play essential roles in the pathogenesis of Alzheimer's disease (AD). Amide proton transfer (APT) imaging, as an imaging modality to detect tissue protein, has shown promising features for the diagnosis of AD disease. In this study, we chose 10 AD model rats as the experimental group and 10 sham-operated rats as the control group. All the rats underwent a Y-maze test before APT image acquisition, using saturation with frequency alternating RF irradiation (APTSAFARI) method on a 7.0 T animal MRI scanner. Compared with the control group, APT (3.5 ppm) values of brain were significantly reduced in AD models (p < 0.002). The APTSAFARI imaging is more significant than APT imaging (p < 0.0001). AD model mice showed spatial learning and memory loss in the Y-maze experiment. In addition, there was significant neuronal loss in the hippocampal CA1 region and cortex compared with sham-operated rats. In conclusion, we demonstrated that APT imaging could potentially provide molecular biomarkers for the non-invasive diagnosis of AD. APTSAFARI MRI could be used as an effective tool to improve the accuracy of diagnosis of AD compared with conventional APT imaging.
Keywords: Alzheimer’s disease; amide proton transfer; chemical exchange saturation transfer; magnetic resonance imaging; saturation with alternating frequency RF irradiation.
Figures






Similar articles
-
Influences of experimental parameters on chemical exchange saturation transfer (CEST) metrics of brain tumors using animal models at 4.7T.Magn Reson Med. 2019 Jan;81(1):316-330. doi: 10.1002/mrm.27389. Epub 2018 Aug 19. Magn Reson Med. 2019. PMID: 30125383 Free PMC article.
-
Mapping Exchangeable Protons to Monitor Protein Alterations in the Brain of an Alzheimer's Disease Mouse Model by Using MRI.Curr Alzheimer Res. 2018;15(14):1343-1353. doi: 10.2174/1567205015666180911143518. Curr Alzheimer Res. 2018. PMID: 30207233
-
Amide proton transfer magnetic resonance imaging of Alzheimer's disease at 3.0 Tesla: a preliminary study.Chin Med J (Engl). 2015 Mar 5;128(5):615-9. doi: 10.4103/0366-6999.151658. Chin Med J (Engl). 2015. PMID: 25698192 Free PMC article.
-
Clinical translation of amide proton transfer (APT) MRI for ischemic stroke: a systematic review (2003-2020).Quant Imaging Med Surg. 2021 Aug;11(8):3797-3811. doi: 10.21037/qims-20-1339. Quant Imaging Med Surg. 2021. PMID: 34341751 Free PMC article. Review.
-
Amide proton transfer imaging of tumors: theory, clinical applications, pitfalls, and future directions.Jpn J Radiol. 2019 Feb;37(2):109-116. doi: 10.1007/s11604-018-0787-3. Epub 2018 Oct 19. Jpn J Radiol. 2019. PMID: 30341472 Review.
Cited by
-
A Multimodal MR Imaging Study of the Effect of Hippocampal Damage on Affective and Cognitive Functions in a Rat Model of Chronic Exposure to a Plateau Environment.Neurochem Res. 2022 Apr;47(4):979-1000. doi: 10.1007/s11064-021-03498-5. Epub 2022 Jan 4. Neurochem Res. 2022. PMID: 34981302 Free PMC article.
-
Saturation Transfer MRI for Detection of Metabolic and Microstructural Impairments Underlying Neurodegeneration in Alzheimer's Disease.Brain Sci. 2021 Dec 30;12(1):53. doi: 10.3390/brainsci12010053. Brain Sci. 2021. PMID: 35053797 Free PMC article. Review.
-
Moyamoya disease: A human model for chronic hypoperfusion and intervention in Alzheimer's disease.Alzheimers Dement (N Y). 2022 Apr 6;8(1):e12285. doi: 10.1002/trc2.12285. eCollection 2022. Alzheimers Dement (N Y). 2022. PMID: 35415209 Free PMC article.
-
Isolation of amide proton transfer effect and relayed nuclear Overhauser enhancement effect at -3.5ppm using CEST with double saturation powers.Magn Reson Med. 2023 Sep;90(3):1025-1040. doi: 10.1002/mrm.29691. Epub 2023 May 8. Magn Reson Med. 2023. PMID: 37154382 Free PMC article.
-
Whole Brain Amide Proton Transfer Weighted Imaging in Children With Obstructive Sleep Apnea.Brain Behav. 2025 Sep;15(9):e70808. doi: 10.1002/brb3.70808. Brain Behav. 2025. PMID: 40891204 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

