Anakinra Drug Retention Rate and Predictive Factors of Long-Term Response in Systemic Juvenile Idiopathic Arthritis and Adult Onset Still Disease
- PMID: 31507416
- PMCID: PMC6715768
- DOI: 10.3389/fphar.2019.00918
Anakinra Drug Retention Rate and Predictive Factors of Long-Term Response in Systemic Juvenile Idiopathic Arthritis and Adult Onset Still Disease
Abstract
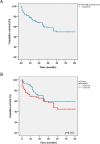
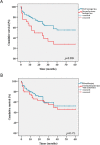
Background and Objective: Only a few studies have reported long-term efficacy of interleukin (IL)-1 inhibition in systemic juvenile idiopathic arthritis (sJIA) and adult-onset Still disease (AOSD). Herein we report on the effectiveness of anakinra (ANA), expressed in terms of drug retention rate (DRR), and evaluate the predictive factors of drug survival in a cohort of patients with sJIA and AOSD. Patients and Methods: This is a multicenter study reviewing retrospectively the medical records from 61 patients with sJIA and 76 with AOSD, all treated with ANA in 25 Italian tertiary referral centers. Results: The cumulative retention rate of ANA at 12-, 24-, 48-, and 60-month of follow-up was 74.3%, 62.9%, 49.4%, and 49.4%, respectively, without any significant differences between sJIA and AOSD patients (p = 0.164), and between patients treated in monotherapy compared with the subgroup coadministered with conventional disease-modifying antirheumatic drugs (cDMARDs) (p = 0.473). On the other hand, a significant difference in DRR was found between biologic-naïve patients and those previously treated with biotechnologic drugs (p = 0.009), which persisted even after adjustment for pathology (p = 0.013). In the regression analysis, patients experiencing adverse events (AEs) {hazards ratio (HR) = 3.029 [confidence interval (CI) 1.750-5.242], p < 0.0001} and those previously treated with other biologic agents [HR = 1.818 (CI 1.007-3.282), p = 0.047] were associated with a higher HR of ANA discontinuation. The median treatment delay was significantly higher among patients discontinuing ANA (p < 0.0001). Significant corticosteroid-sparing (p = 0.033) and cDMARD-sparing effects (p < 0.0001) were also recorded. Less than one-third of our cohort developed AEs, and 85% were deemed mild in nature, with 70% of them involving the skin. Conclusions: Our findings display an overall excellent DRR of ANA on the long run for both sJIA and AOSD, that may be further optimized by closely monitoring patient's safety issues and employing this IL-1 inhibitor as a first-line biologic as early as possible. Moreover, ANA allowed a significant drug-sparing effect and showed an overall good safety profile.
Keywords: adult onset Still disease; anakinra; drug retention rate; innovative biotechnologies; interleukin 1-beta; personalized medicine; systemic juvenile idiopathic arthritis.
Figures
Similar articles
-
Long-Term Retention Rate of Anakinra in Adult Onset Still's Disease and Predictive Factors for Treatment Response.Front Pharmacol. 2019 Apr 2;10:296. doi: 10.3389/fphar.2019.00296. eCollection 2019. Front Pharmacol. 2019. PMID: 31001115 Free PMC article.
-
Drug Retention Rate and Predictive Factors of Drug Survival for Interleukin-1 Inhibitors in Systemic Juvenile Idiopathic Arthritis.Front Pharmacol. 2019 Jan 8;9:1526. doi: 10.3389/fphar.2018.01526. eCollection 2018. Front Pharmacol. 2019. PMID: 30670972 Free PMC article.
-
Response to Interleukin-1 Inhibitors in 140 Italian Patients with Adult-Onset Still's Disease: A Multicentre Retrospective Observational Study.Front Pharmacol. 2017 Jun 13;8:369. doi: 10.3389/fphar.2017.00369. eCollection 2017. Front Pharmacol. 2017. PMID: 28659802 Free PMC article.
-
The role of IL-1 inhibition in systemic juvenile idiopathic arthritis: current status and future perspectives.Drug Des Devel Ther. 2018 Jun 8;12:1633-1643. doi: 10.2147/DDDT.S114532. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 29922038 Free PMC article. Review.
-
Anakinra in children and adults with Still's disease.Rheumatology (Oxford). 2019 Nov 1;58(Suppl 6):vi9-vi22. doi: 10.1093/rheumatology/kez350. Rheumatology (Oxford). 2019. PMID: 31769856 Free PMC article. Review.
Cited by
-
Successful treatment of interstitial pneumonitis with anakinra in a patient with adult-onset Still's disease.Eur J Hosp Pharm. 2021 Nov;28(6):346-349. doi: 10.1136/ejhpharm-2020-002377. Epub 2020 Aug 11. Eur J Hosp Pharm. 2021. PMID: 32788403 Free PMC article.
-
Serum Heparin-Binding Protein as a Potential Biomarker to Distinguish Adult-Onset Still's Disease From Sepsis.Front Immunol. 2021 Mar 31;12:654811. doi: 10.3389/fimmu.2021.654811. eCollection 2021. Front Immunol. 2021. PMID: 33868298 Free PMC article.
-
Cardiovascular involvement in children with COVID-19 temporally related multisystem inflammatory syndrome (MIS-C): can cardiac magnetic resonance arrive to the heart of the problem?Ital J Pediatr. 2024 May 3;50(1):91. doi: 10.1186/s13052-024-01658-1. Ital J Pediatr. 2024. PMID: 38702753 Free PMC article.
-
Advancing multidisciplinary management of pediatric hyperinflammatory disorders.Front Pediatr. 2025 Apr 30;13:1553861. doi: 10.3389/fped.2025.1553861. eCollection 2025. Front Pediatr. 2025. PMID: 40370972 Free PMC article. Review.
-
MIS-C, inherited metabolic diseases and methylmalonic acidemia: a case report and review of the literature.Ital J Pediatr. 2025 Jul 1;51(1):202. doi: 10.1186/s13052-025-02052-1. Ital J Pediatr. 2025. PMID: 40597391 Free PMC article. Review.
References
-
- Colafrancesco S., Priori R., Valesini G., Argolini L., Baldissera E., Bartoloni E., et al. (2017). Response to interleukin-1 inhibitors in 140 Italian patients with adult-onset Still’s disease: a multicentre retrospective observational study. Front. Pharmacol. 8, 369. 10.3389/fphar.2017.00369 - DOI - PMC - PubMed

