Imaging the Dynamic Interaction Between Sprouting Microvessels and the Extracellular Matrix
- PMID: 31507428
- PMCID: PMC6713949
- DOI: 10.3389/fphys.2019.01011
Imaging the Dynamic Interaction Between Sprouting Microvessels and the Extracellular Matrix
Abstract
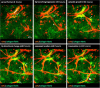
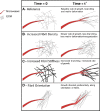
Thorough understanding of growth and evolution of tissue vasculature is fundamental to many fields of medicine including cancer therapy, wound healing, and tissue engineering. Angiogenesis, the growth of new vessels from existing ones, is dynamically influenced by a variety of environmental factors, including mechanical and biophysical factors, chemotactic factors, proteolysis, and interaction with stromal cells. Yet, dynamic interactions between neovessels and their environment are difficult to study with traditional fixed time imaging techniques. Advancements in imaging technologies permit time-series and volumetric imaging, affording the ability to visualize microvessel growth over 3D space and time. Time-lapse imaging has led to more informative investigations of angiogenesis. The environmental factors implicated in angiogenesis span a wide range of signals. Neovessels advance through stromal matrices by forming attachments and pulling and pushing on their microenvironment, reorganizing matrix fibers, and inducing large deformations of the surrounding stroma. Concurrently, neovessels secrete proteolytic enzymes to degrade their basement membrane, create space for new vessels to grow, and release matrix-bound cytokines. Growing neovessels also respond to a host of soluble and matrix-bound growth factors, and display preferential growth along a cytokine gradient. Lastly, stromal cells such as macrophages and mesenchymal stem cells (MSCs) interact directly with neovessels and their surrounding matrix to facilitate sprouting, vessel fusion, and tissue remodeling. This review highlights how time-lapse imaging techniques advanced our understanding of the interaction of blood vessels with their environment during sprouting angiogenesis. The technology provides means to characterize the evolution of microvessel behavior, providing new insights and holding great promise for further research on the process of angiogenesis.
Keywords: angiogenesis; extracellular matrix; neovessels; time-series imaging; vascular networks.
Figures







Similar articles
-
Large-scale time series microscopy of neovessel growth during angiogenesis.Angiogenesis. 2015 Jul;18(3):219-32. doi: 10.1007/s10456-015-9461-x. Epub 2015 Mar 21. Angiogenesis. 2015. PMID: 25795217 Free PMC article.
-
Mechanical interaction of angiogenic microvessels with the extracellular matrix.J Biomech Eng. 2014 Feb;136(2):021001. doi: 10.1115/1.4026471. J Biomech Eng. 2014. PMID: 24441831 Free PMC article. Review.
-
Formation of microvascular networks: role of stromal interactions directing angiogenic growth.Microcirculation. 2014 May;21(4):278-89. doi: 10.1111/micc.12115. Microcirculation. 2014. PMID: 24447042 Free PMC article. Review.
-
2.5D Model for Ex Vivo Mechanical Characterization of Sprouting Angiogenesis in Living Tissue.J Vis Exp. 2025 Feb 28;(216). doi: 10.3791/67641. J Vis Exp. 2025. PMID: 40095983
-
A coupled model of neovessel growth and matrix mechanics describes and predicts angiogenesis in vitro.Biomech Model Mechanobiol. 2015 Aug;14(4):767-82. doi: 10.1007/s10237-014-0635-z. Epub 2014 Nov 28. Biomech Model Mechanobiol. 2015. PMID: 25429840 Free PMC article.
Cited by
-
Endothelial cell polarity and extracellular matrix composition require functional ATP6AP2 during developmental and pathological angiogenesis.JCI Insight. 2022 Oct 10;7(19):e154379. doi: 10.1172/jci.insight.154379. JCI Insight. 2022. PMID: 35998033 Free PMC article.
-
Extracellular Vesicles From Notch Activated Cardiac Mesenchymal Stem Cells Promote Myocyte Proliferation and Neovasculogenesis.Front Cell Dev Biol. 2020 Feb 21;8:11. doi: 10.3389/fcell.2020.00011. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32154243 Free PMC article.
-
Generation of Connective Tissue-Free Microvascular Fragment Isolates from Subcutaneous Fat Tissue of Obese Mice.Tissue Eng Regen Med. 2023 Dec;20(7):1079-1090. doi: 10.1007/s13770-023-00571-8. Epub 2023 Oct 2. Tissue Eng Regen Med. 2023. PMID: 37783934 Free PMC article.
-
Dynamic Biophysical Cues Near the Tip Cell Microenvironment Provide Distinct Guidance Signals to Angiogenic Neovessels.Ann Biomed Eng. 2023 Aug;51(8):1835-1846. doi: 10.1007/s10439-023-03202-4. Epub 2023 May 6. Ann Biomed Eng. 2023. PMID: 37149511
-
Computational Evaluation of Improved HIPEC Drug Delivery Kinetics via Bevacizumab-Induced Vascular Normalization.Pharmaceutics. 2025 Jan 23;17(2):155. doi: 10.3390/pharmaceutics17020155. Pharmaceutics. 2025. PMID: 40006522 Free PMC article.
References
-
- Ardi V. C., Van Den Steen P. E., Opdenakker G., Schweighofer B., Deryugina E. I., Quigley J. P. (2009). Neutrophil MMP-9 proenzyme, unencumbered by TIMP-1, undergoes efficient activation in vivo and catalytically induces angiogenesis via a basic fibroblast growth factor (FGF-2)/FGFR-2 pathway. J. Biol. Chem. 284, 25854–25866. 10.1074/jbc.M109.033472, PMID: - DOI - PMC - PubMed
-
- Bacharach E., Itin A., Keshet E. (1998). Apposition-dependent induction of plasminogen activator inhibitor type 1 expression: a mechanism for balancing pericellular proteolysis during angiogenesis. Blood 92, 939–945. - PubMed
-
- Barkefors I., Le Jan S., Jakobsson L., Hejll E., Carlson G., Johansson H., et al. (2008). Endothelial cell migration in stable gradients of vascular endothelial growth factor A and fibroblast growth factor 2: effects on chemotaxis and chemokinesis. J. Biol. Chem. 283, 13905–13912. 10.1074/jbc.M704917200 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

