Interrelationship Between Contractility, Protein Synthesis and Metabolism in Mantle of Juvenile Cuttlefish (Sepia officinalis)
- PMID: 31507433
- PMCID: PMC6716058
- DOI: 10.3389/fphys.2019.01051
Interrelationship Between Contractility, Protein Synthesis and Metabolism in Mantle of Juvenile Cuttlefish (Sepia officinalis)
Abstract
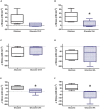
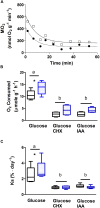
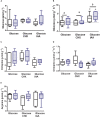
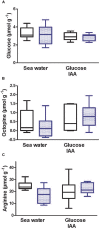
Young juvenile cuttlefish (Sepia officinalis) can grow at rates as high as 12% body weight per day. How the metabolic demands of such a massive growth rate impacts muscle performance that competes for ATP is unknown. Here, we integrate aspects of contractility, protein synthesis, and energy metabolism in mantle of specimens weighing 1.1 g to lend insight into the processes. Isolated mantle muscle preparations were electrically stimulated and isometric force development monitored. Preparations were forced to contract at 3 Hz for 30 s to simulate a jetting event. We then measured oxygen consumption, glucose uptake and protein synthesis in the hour following the stimulation. Protein synthesis was inhibited with cycloheximide and glycolysis was inhibited with iodoacetic acid in a subset of samples. Inhibition of protein synthesis impaired contractility and decreased oxygen consumption. An intact protein synthesis is required to maintain contractility possibly due to rapidly turning over proteins. At least, 41% of whole animal ṀO2 is used to support protein synthesis in mantle, while the cost of protein synthesis (50 μmol O2 mg protein-1) in mantle was in the range reported for other aquatic ectotherms. A single jetting challenge stimulated protein synthesis by approximately 25% (2.51-3.12% day-1) over a 1 h post contractile period, a similar response to that which occurs in mammalian skeletal muscle. Aerobic metabolism was not supported by extracellular glucose leading to the contention that at this life stage either glycogen or amino acids are catabolized. Regardless, an intact glycolysis is required to support contractile performance and protein synthesis in resting muscle. It is proposed that glycolysis is needed to maintain intracellular ionic gradients. Intracellular glucose at approximately 3 mmol L-1 was higher than the 1 mmol L-1 glucose in the bathing medium suggesting an active glucose transport mechanism. Octopine did not accumulate during a single physiologically relevant jetting challenge; however, octopine accumulation increased following a stress that is sufficient to lower Arg-P and increase free arginine.
Keywords: anaerobic metabolism; cycloheximide; glucose; iodoacetic acid; jetting; octopine.
Figures

Similar articles
-
Hypoxic Induced Decrease in Oxygen Consumption in Cuttlefish (Sepia officinalis) Is Associated with Minor Increases in Mantle Octopine but No Changes in Markers of Protein Turnover.Front Physiol. 2017 May 26;8:344. doi: 10.3389/fphys.2017.00344. eCollection 2017. Front Physiol. 2017. PMID: 28603503 Free PMC article.
-
Critical temperatures in the cephalopod Sepia officinalis investigated using in vivo 31P NMR spectroscopy.J Exp Biol. 2006 Mar;209(Pt 5):891-906. doi: 10.1242/jeb.02054. J Exp Biol. 2006. PMID: 16481578
-
Taurine depresses cardiac contractility and enhances systemic heart glucose utilization in the cuttlefish, Sepia officinalis.J Comp Physiol B. 2016 Feb;186(2):215-27. doi: 10.1007/s00360-015-0946-0. Epub 2015 Dec 7. J Comp Physiol B. 2016. PMID: 26644087
-
Metabolism and insulin signaling in common metabolic disorders and inherited insulin resistance.Dan Med J. 2014 Jul;61(7):B4890. Dan Med J. 2014. PMID: 25123125 Review.
-
Studies of gene expression and activity of hexokinase, phosphofructokinase and glycogen synthase in human skeletal muscle in states of altered insulin-stimulated glucose metabolism.Dan Med Bull. 1999 Feb;46(1):13-34. Dan Med Bull. 1999. PMID: 10081651 Review.
Cited by
-
The mechanical properties of the mantle muscle of European cuttlefish (Sepia officinalis).J Exp Biol. 2022 Dec 1;225(23):jeb244977. doi: 10.1242/jeb.244977. Epub 2022 Dec 15. J Exp Biol. 2022. PMID: 36416079 Free PMC article.
-
Food availability modulates the combined effects of ocean acidification and warming on fish growth.Sci Rep. 2020 Feb 11;10(1):2338. doi: 10.1038/s41598-020-58846-2. Sci Rep. 2020. PMID: 32047178 Free PMC article.
References
-
- Biolo G., Maggi S. P., Williams B. D., Tipton K. D., Wolfe R. R. (1995). Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am. J. Physiol. 268 E514–E520. - PubMed
-
- Capaz J. C., Tunnah L., MacCormack T. J., Lamarre S. G., Sykes A. V., Driedzic W. R. (2017). Hypoxic induced decrease in oxygen consumption in cuttlefish (Sepia officinalis) is associated with minor increases in mantle octopine but no changes in markers of protein turnover. Front. Physiol. 8:344. 10.3389/fphys.2017.00344 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

