TLR2-Deficiency Promotes Prenatal LPS Exposure-Induced Offspring Hyperlipidemia
- PMID: 31507457
- PMCID: PMC6713936
- DOI: 10.3389/fphys.2019.01102
TLR2-Deficiency Promotes Prenatal LPS Exposure-Induced Offspring Hyperlipidemia
Abstract
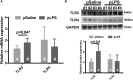
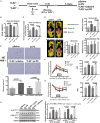
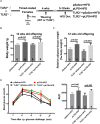
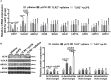
Toll-like receptor 2 (TLR2), which recognizes several lipopeptides and transduces inflammatory signaling, promotes the pathogenesis of diet-induced dyslipidemia and obesity. TLR2-deficient mice were shown to have improved insulin sensitivity and reduced diet-induced metabolic syndrome. Previous studies demonstrated that prenatal lipopolysaccharide (LPS) exposure causes dyslipidemia accompanied by increased body weight and insulin resistance in offspring. To determine whether TLRs are involved in this complex abnormal phenotype, we analyzed TLR2 and TLR4 expression levels in adipose tissues from offspring with prenatal LPS-exposure (offspring-pLPS) and compared these levels to those of control offspring with prenatal saline-exposure (offspring-pSaline). TLR2 expression was specifically upregulated in the adipose tissue of offspring-pLPS mice. However, unexpectedly, TLR2-deficient offspring-pLPS mice not only presented with an abnormal phenotype comparable to that of wild-type offspring-pLPS mice but also exhibited significantly more severe hyperlipidemia. Our further analyses revealed a dramatic upregulation of TLR4 expression and overactivation of the TLR4/Myd88 signaling pathway in TLR2-deficient offspring-pLPS adipose tissue. Our finding suggests a compensatory genetic interaction between TLR2 and TLR4 in the context of prenatal inflammatory stimulation, and this interaction likely contributes to the prenatal inflammation-induced hyperlipidemia and lipid overload-induced obesity, thus providing a potential mechanism for the fetal origin of adult metabolic diseases.
Keywords: TLR2; TLR4; VLDLR; hyperlipidemia; prenatal lipopolysaccharide stimulation.
Figures



Similar articles
-
Prenatal Inflammatory Exposure Predisposes Offspring to Chronic Kidney Diseases Via the Activation of the eIF2α-ATF4 Pathway.Inflammation. 2025 Apr;48(2):747-759. doi: 10.1007/s10753-024-02084-5. Epub 2024 Jun 24. Inflammation. 2025. PMID: 38913145
-
Induction of tolerance to lipopolysaccharide and mycobacterial components in Chinese hamster ovary/CD14 cells is not affected by overexpression of Toll-like receptors 2 or 4.J Immunol. 2001 Aug 15;167(4):2257-67. doi: 10.4049/jimmunol.167.4.2257. J Immunol. 2001. PMID: 11490013
-
Mice lacking myeloid differentiation factor 88 display profound defects in host resistance and immune responses to Mycobacterium avium infection not exhibited by Toll-like receptor 2 (TLR2)- and TLR4-deficient animals.J Immunol. 2003 Nov 1;171(9):4758-64. doi: 10.4049/jimmunol.171.9.4758. J Immunol. 2003. PMID: 14568952
-
Increased lipopolysaccharide sensitivity in alcoholic fatty livers is independent of leptin deficiency and toll-like receptor 4 (TLR4) or TLR2 mRNA expression.Alcohol Clin Exp Res. 2005 Jun;29(6):1018-26. doi: 10.1097/01.alc.0000167744.60838.4a. Alcohol Clin Exp Res. 2005. PMID: 15976528
-
The Janus face of Bartonella quintana recognition by Toll-like receptors (TLRs): a review.Eur Cytokine Netw. 2008 Sep;19(3):113-8. doi: 10.1684/ecn.2008.0128. Epub 2008 Sep 8. Eur Cytokine Netw. 2008. PMID: 18775802 Review.
Cited by
-
Palmitate Stimulates Expression of the von Willebrand Factor and Modulates Toll-like Receptors Level and Activity in Human Umbilical Vein Endothelial Cells (HUVECs).Int J Mol Sci. 2023 Dec 23;25(1):254. doi: 10.3390/ijms25010254. Int J Mol Sci. 2023. PMID: 38203423 Free PMC article.
-
Targets of statins intervention in LDL-C metabolism: Gut microbiota.Front Cardiovasc Med. 2022 Sep 8;9:972603. doi: 10.3389/fcvm.2022.972603. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36158845 Free PMC article. Review.
-
Prenatal Inflammatory Exposure Predisposes Offspring to Chronic Kidney Diseases Via the Activation of the eIF2α-ATF4 Pathway.Inflammation. 2025 Apr;48(2):747-759. doi: 10.1007/s10753-024-02084-5. Epub 2024 Jun 24. Inflammation. 2025. PMID: 38913145
-
Potential Mechanisms of Gut-Derived Extracellular Vesicle Participation in Glucose and Lipid Homeostasis.Genes (Basel). 2022 Oct 28;13(11):1964. doi: 10.3390/genes13111964. Genes (Basel). 2022. PMID: 36360201 Free PMC article. Review.
-
Employing single-cell RNA sequencing coupled with an array of bioinformatics approaches to ascertain the shared genetic characteristics between osteoporosis and obesity.Bone Joint Res. 2024 Oct 16;13(10):573-587. doi: 10.1302/2046-3758.1310.BJR-2023-0366.R1. Bone Joint Res. 2024. PMID: 39412449 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

