Intrathecal Baclofen in Hereditary Spastic Paraparesis
- PMID: 31507512
- PMCID: PMC6716054
- DOI: 10.3389/fneur.2019.00901
Intrathecal Baclofen in Hereditary Spastic Paraparesis
Abstract
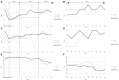
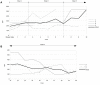
Introduction: Treatment with intrathecal baclofen (ITB) is a therapeutic option in the management of severe spasticity in patients with hereditary spastic paraparesis (HSP). However, information on the impact of ITB on the natural course of disease, especially the effect of ITB on functional parameters over time is limited. Materials and Methods: We evaluated seven patients with HSP retrospectively who were treated with an ITB device. The following parameters were measured before (pre-implantation) and after implantation (post-implantation) of the ITB device at steady state dosage of ITB and annually until last follow-up: modified Ashworth Scale, Reflex Scale, modified Rankin Scale, and Rivermead Mobility Index. The ITB dosages were assessed after reaching steady state as well as annually until last follow-up. Results: The ITB device was implanted 13 ± 6 (range 9-16) years after diagnosis of HSP on average. Severe spasticity was controlled in all patients by a mean baclofen dosage of 188 ± 60 (range 145-230) μg per day at steady state post-implantation. The modified Ashworth Scale improved significantly from 3 (interquartile range [IQR] 3-3.25) to 1 (IQR 1-1.25; p = 0.046), as did the Reflex Scale from 5 (IQR 4.75-5) to 3 (IQR 2.75-3; p = 0.046) at steady state dosage of ITB. The modified Rankin Scale improved from 2 (IQR 2-2) to 1 (IQR 1-1.5; p = 0.083) and the Rivermead Mobility Index remained 14 (IQR 13.5-14 pre-implantation, IQR 14-14 post-implantation; p = 0.18). Post-implantation, spasticity improved for 2-3 years, followed by a stable phase of ambulatory and other mobility functions for 4-5 years. Thereafter, the maintenance or progressive loss of mobility depended on individual courses of the disease. No ITB-related severe side effects occurred. Discussion: Our data further support the role of ITB in the treatment of severe spasticity in patients with deteriorated walking performance suffering HSP. ITB therapy may initially improve spasticity and stabilize mobility functions for the first 6-8 years in patients with HSP.
Keywords: device implantation; hereditary spastic paraparesis; intrathecal baclofen; spasticity; sporadic spastic paraparesis.
Figures
Similar articles
-
Intrathecal Baclofen Trial Before Device Implantation: 12-Year Experience With Continuous Administration.Arch Phys Med Rehabil. 2019 May;100(5):837-843. doi: 10.1016/j.apmr.2018.09.124. Epub 2018 Oct 26. Arch Phys Med Rehabil. 2019. PMID: 31030729
-
Improved gait performance in a patient with hereditary spastic paraplegia after a continuous intrathecal baclofen test infusion and subsequent pump implantation: a case report.Arch Phys Med Rehabil. 2015 Jun;96(6):1166-9. doi: 10.1016/j.apmr.2015.01.012. Epub 2015 Jan 24. Arch Phys Med Rehabil. 2015. PMID: 25626112
-
Intrathecal baclofen for the management of hereditary spastic paraparesis: a systematic review.Int J Rehabil Res. 2024 Mar 1;47(1):3-9. doi: 10.1097/MRR.0000000000000607. Epub 2024 Jan 20. Int J Rehabil Res. 2024. PMID: 38251093
-
Optimizing Physical Assessment in Intrathecal Baclofen Screening Trials for High-Performing Patients With Spastic Paraparesis.Neuromodulation. 2025 Jan 7:S1094-7159(24)01267-4. doi: 10.1016/j.neurom.2024.12.001. Online ahead of print. Neuromodulation. 2025. PMID: 39772339
-
[Intrathecal baclofen treatment for spasticity in childhood . initial report of long-term follow up in Japan].No To Hattatsu. 2014 May;46(3):179-86. No To Hattatsu. 2014. PMID: 24902335 Review. Japanese.
Cited by
-
Characteristics of Changes in Intrathecal Baclofen Dosage over Time due to Causative Disease.Neurol Med Chir (Tokyo). 2023 Dec 15;63(12):535-541. doi: 10.2176/jns-nmc.2022-0359. Epub 2023 Sep 23. Neurol Med Chir (Tokyo). 2023. PMID: 37743509 Free PMC article.
-
Novel genetic variant in hereditary spastic paraparesis.BMJ Case Rep. 2024 Apr 17;17(4):e252396. doi: 10.1136/bcr-2022-252396. BMJ Case Rep. 2024. PMID: 38631813
-
Current techniques for the treatment of spasticity and their effectiveness.EFORT Open Rev. 2025 May 5;10(5):237-249. doi: 10.1530/EOR-2024-0156. EFORT Open Rev. 2025. PMID: 40326554 Free PMC article.
-
Surgical treatment options for spasticity in children and adolescents with hereditary spastic paraplegia.Childs Nerv Syst. 2024 Mar;40(3):855-861. doi: 10.1007/s00381-023-06159-w. Epub 2023 Oct 3. Childs Nerv Syst. 2024. PMID: 37783799 Free PMC article.
-
Hereditary spastic paraplegia: Novel insights into the pathogenesis and management.SAGE Open Med. 2023 Dec 29;12:20503121231221941. doi: 10.1177/20503121231221941. eCollection 2024. SAGE Open Med. 2023. PMID: 38162912 Free PMC article. Review.
References
-
- Bundey S. (ed.). Genetics and Neurology. Edinburgh: Churchill Livingstone; (1992).
LinkOut - more resources
Full Text Sources

