Light-Emitting Diode Photobiomodulation After Cerebral Ischemia
- PMID: 31507516
- PMCID: PMC6713875
- DOI: 10.3389/fneur.2019.00911
Light-Emitting Diode Photobiomodulation After Cerebral Ischemia
Abstract
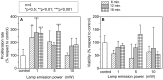
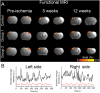
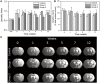
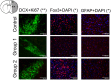
Photobiomodulation (PBM) therapy is a promising therapeutic approach for several pathologies, including stroke. The biological effects of PBM for the treatment of cerebral ischemia have previously been explored as a neuroprotective strategy using different light sources, wavelengths, and incident light powers. However, the capability of PBM as a novel alternative therapy to stimulate the recovery of the injured neuronal tissue after ischemic stroke has been poorly explored. The aim of this study was to investigate the low-level light irradiation therapy by using Light Emitting Diodes (LEDs) as potential therapeutic strategy for stroke. The LED photobiomodulation (continuous wave, 830 nm, 0.2-0.6 J/cm2) was firstly evaluated at different energy densities in C17.2 immortalized mouse neural progenitor cell lines, in order to observe if this treatment had any effect on cells, in terms of proliferation and viability. Then, the PBM-LED effect (continuous wave, 830 nm, 0.28 J/cm2 at brain cortex) on long-term recovery (12 weeks) was analyzed in ischemic animal model by means lesion reduction, behavioral deficits, and functional magnetic resonance imaging (fMRI). Analysis of cellular proliferation after PBM was significantly increased (1 mW) in all different exposure times used; however, this effect could not be replicated in vivo experimental conditions, as PBM did not show an infarct reduction or functional recovery. Despite the promising therapeutic effect described for PBM, further preclinical studies are necessary to optimize the therapeutic window of this novel therapy, in terms of the mechanism associated to neurorecovery and to reduce the risk of failure in futures clinical trials.
Keywords: animal model; functional recovery; intracerebral hemorrhage; ischemic stroke; magnetic resonance imaging; photobiomodulation therapy.
Figures
References
LinkOut - more resources
Full Text Sources

