Role of Aldosterone and Mineralocorticoid Receptor in Cardiovascular Aging
- PMID: 31507534
- PMCID: PMC6716354
- DOI: 10.3389/fendo.2019.00584
Role of Aldosterone and Mineralocorticoid Receptor in Cardiovascular Aging
Abstract
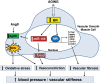
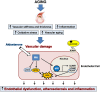
The mineralocorticoid receptor (MR) was originally identified as a regulator of blood pressure, able to modulate renal sodium handling in response to its principal ligand aldosterone. MR is expressed in several extra-renal tissues, including the heart, vasculature, and adipose tissue. More recent studies have shown that extra-renal MR plays a relevant role in the control of cardiovascular and metabolic functions and has recently been implicated in the pathophysiology of aging. MR activation promotes vasoconstriction and acts as a potent pro-fibrotic agent in cardiovascular remodeling. Aging is associated with increased arterial stiffness and vascular tone, and modifications of arterial structure and function are responsible for these alterations. MR activation contributes to increase blood pressure with aging by regulating myogenic tone, vasoconstriction, and vascular oxidative stress. Importantly, aging represents an important contributor to the increased prevalence of cardiometabolic syndrome. In the elderly, dysregulation of MR signaling is associated with hypertension, obesity, and diabetes, representing an important cause of increased cardiovascular risk. Clinical use of MR antagonists is limited by the adverse effects induced by MR blockade in the kidney, raising the risk of hyperkalaemia in older patients with reduced renal function. Therefore, there is an unmet need for the enhanced understanding of the role of MR in aging and for development of novel specific MR antagonists in the context of cardiovascular rehabilitation in the elderly, in order to reduce relevant side effects.
Keywords: RAAS; endothelial dysfunction; mineralocorticoid receptor; oxidative stress; vascular stiffness.
Figures
Similar articles
-
Mineralocorticoid Receptor and Aldosterone-Related Biomarkers of End-Organ Damage in Cardiometabolic Disease.Biomolecules. 2018 Sep 18;8(3):96. doi: 10.3390/biom8030096. Biomolecules. 2018. PMID: 30231508 Free PMC article. Review.
-
30 YEARS OF THE MINERALOCORTICOID RECEPTOR: The role of the mineralocorticoid receptor in the vasculature.J Endocrinol. 2017 Jul;234(1):T67-T82. doi: 10.1530/JOE-17-0009. J Endocrinol. 2017. PMID: 28634267 Free PMC article. Review.
-
The role of mineralocorticoid receptor signaling in the cross-talk between adipose tissue and the vascular wall.Cardiovasc Res. 2017 Jul 1;113(9):1055-1063. doi: 10.1093/cvr/cvx097. Cardiovasc Res. 2017. PMID: 28838041 Free PMC article. Review.
-
Vascular Consequences of Aldosterone Excess and Mineralocorticoid Receptor Antagonism.Curr Hypertens Rev. 2017;13(1):46-56. doi: 10.2174/1573402113666170228151402. Curr Hypertens Rev. 2017. PMID: 28245785 Review.
-
Posttranslational Modifications of the Mineralocorticoid Receptor and Cardiovascular Aging.Front Mol Biosci. 2021 May 28;8:667990. doi: 10.3389/fmolb.2021.667990. eCollection 2021. Front Mol Biosci. 2021. PMID: 34124152 Free PMC article. Review.
Cited by
-
Mineralocorticoid receptor promotes cardiac macrophage inflammaging.Basic Res Cardiol. 2024 Apr;119(2):243-260. doi: 10.1007/s00395-024-01032-6. Epub 2024 Feb 8. Basic Res Cardiol. 2024. PMID: 38329499 Free PMC article.
-
Mineralocorticoid Receptor Antagonists Mitigate Mitral Regurgitation-Induced Myocardial Dysfunction.Cells. 2022 Sep 3;11(17):2750. doi: 10.3390/cells11172750. Cells. 2022. PMID: 36078158 Free PMC article.
-
Therapeutic targeting of mineralocorticoid receptors in pulmonary hypertension: Insights from basic research.Front Cardiovasc Med. 2023 Jan 30;10:1118516. doi: 10.3389/fcvm.2023.1118516. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 36793473 Free PMC article. Review.
-
Cardiovascular Disease in Obstructive Sleep Apnea: Putative Contributions of Mineralocorticoid Receptors.Int J Mol Sci. 2023 Jan 23;24(3):2245. doi: 10.3390/ijms24032245. Int J Mol Sci. 2023. PMID: 36768567 Free PMC article. Review.
-
Association Between Aldosterone and Hypertension Among Patients With Overt and Subclinical Hypercortisolism.J Endocr Soc. 2022 Oct 31;7(1):bvac167. doi: 10.1210/jendso/bvac167. eCollection 2022 Nov 17. J Endocr Soc. 2022. PMID: 36438547 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

